Safety evaluation of the Ngul be Tara Plant Remedy used for COVID-19 Management in Cameroon
Cite: Akuodor, G.C., Agbor, G.A., Nwibo, S.E., Nwaze, E.O., Pieme, C.A., Peyou-Ndi, M.M. and Torimiro J. (2022). 'Safety evaluation of the Ngul be Tara Plant Remedy used for COVID-19 Management in Cameroon'. The Int. J. Holist. Sci. Innov. Dev., 11(3), pp. 31–46.
Safety evaluation of the Ngul be Tara Plant Remedy used for COVID-19 Management in Cameroon
Godwin C. Akuodor1, Gabriel A. Agbor2, Simon E. Nwibo1, Eric O. Nwaze3, Constant A. Pieme4, Marlyse M. Peyou-Ndi4,5 and Judith Torimiro4,6:
Corresponding author: Godwin Akuodor, goddyakuodor@yahoo.com- Accepted: October 2022 - Published: November 2022
ABSTRACT
Ngul be Tara (NBT), a widely used herbal formula in traditional Cameroun and African medicine, has been used to treat common cold and illnesses including COVID-19, but there was no worldwide published information on its safety. To get more insights into the safety of NBT, its acute and sub-acute toxicity were examined using male and female Swiss Albino mice. Mice were treated with NBT orally at 10, 100, 1000, 1600, 2900, 5000 and 8000 mg /kg body weight for two weeks. In the sub-acute toxicity study, 200 to 400 mg of NBT/kg body weight were administered for 28 days, the latter doses were 10 to 20 times the therapeutic dose. Mortality, clinical signs, body weight changes, food and water consumption, ophthalmologic findings, urinalysis, hematological and biochemical parameters, gross findings, organ weights and histological markers were monitored during the study period. We found no mortality and no abnormality in clinical signs, body weight, and necropsy findings for any of the animals in the acute and sub-acute toxicity study following oral administration of NBT up to 5000 mg/kg body weight. The lethal dose with a 50% mortality rate (LD50) was over 5000 mg/kg. The no-observed adverse effects level (NOAEL) was considered to be 5000 mg/kg of body weight for male and female mice, respectively. These studies, having provided some biochemical safety basis, indicate that the ethnomedical use of NBT in the management of coronavirus diseases is safe.
Key words: Ngul be Tara, COVID19, acute toxicity, plant remedy
1. Introduction
History of medicine reveals that mankind has mainly used plants as the best source of medicine [1]. Over 248,000 species of higher plants have been identified and from these more than 12,000 plants are known to have medicinal properties [2]. The past three decades have seen a significant increase in the use of plant-based medicines with many people across the world relying on them for some part of their primary healthcare [3]. The basis for the medicinal use of the plants is the presence of mixtures of biologically active plant phytochemicals such as alkaloids, glycosides, terpenoids, tannins, and others that may act individually, additively or in synergy to demonstrate an effect which may be useful or harmful to health. These compounds’ dose received results from either acute (short), subacute (medium term) or chronic (long-term) exposure. Indeed, some plants used in traditional medicine or used as food have demonstrated some toxicity effects [4], [5]. The issue of the possible toxic, genotoxic and/or mutagenic effects of plants has been highlighted in reviews [6].
However, in traditional medicine, plants with toxic constituents are usually known and are avoided or used cautiously in herbal product formulations by experimented practitioners. Even when potentially toxic plants are employed in medicinal products, they are employed below toxic levels and hence, if at all, hardly result in any fatality when administered by professional practitioners or experienced people [7]. Thus, in many cultural settings, toxic fatalities are rare when appropriate selection is realized by professionals. While thousands of people die each year from even supposedly “safe” over-the-counter remedies, deaths or hospitalizations due to herbs are hard to find [7].
Plants are used for the prevention and treatment of several ailments worldwide. Needless to say, COVID-19 is one of the diseases addressed with traditional medicines in several countries. Indeed, while most of the COVID-19 affected countries in Europe and America are relying mostly on conventional drugs, South-East Asia and in particular, China where the COVID-19 pandemic appear to have originated, has adequate documentation of successful outcomes following the integration of Traditional Chinese Medicine (TCM) with conventional medicines in COVID19 management [8]. In Africa, the least affected continent, most countries also rely on traditional medicines to address the COVID-19 pandemic. However, while pharmacological studies have been conducted to ascertain the traditional uses of medicinal plants in coronavirus management, not as much plant formula have been thoroughly evaluated for their potential detrimental effect. In general, reports of efficacy are, by far, more numerous than those on toxicity [9], [10]. There is, therefore, a need to further investigate of herbal remedies such as NBT, to incorporate the observations of short and long-term toxicity manifestations and to ensure effectual open communication of such findings.
The NBT remedy is classified as nationally validated category II improved traditional medicine used for COVID-19 management, and is prepared from 4 major components namely Alstonia boonei, Enantia chlorantha, Guibourtia tessùannii and Euphorba hirta. The various ethnomedicinal, chemical, pharmacological, and toxicological properties of Alstoniaboonei were reviewed and the profile revealed it is useful in the treatment and management of several illnesses [11]. Besides, the plant is considered non-toxic at therapeutic doses. The LD50 was estimated to be 6 170 mg/kg, doses higher than this will have a lethal effect on 50% of the population [12]. Extracts of E. chlorantha, is considered nontoxic in acute intake up to 5000 mg/kg, with LD50 >5000 mg/kg [13]. Studies showed that the aqueous extract of G. tessmannii has low toxicity intraperitoneally and no toxicity via oral intake. A LD50 of 328.78 mg/kg of body weight was obtained by intra peritoneal route and a LD50 over 5000 mg/kg of body weight was calculated by oral route [14]. Euphorbia hirta: extracts at a single dose of 5000mg/kg did not produce treatment related signs of toxicity or mortality in any of the animals tested during the 14-day observation period. Therefore, the LD50 of this plant was estimated to be more than 5000mg/kg of body weight [15].
Having nontoxic ingredient does not necessarily imply that mixing them will provide a safe mixture. Despite the ancestral and millennium long reported use of major and minor ingredients of NBT, the mixture needed not only to be analyzed, but also tested for its innocuity. We set out to get more evidence into the safety of the African remedy NBT
2. Materials and Methods
2.1. Experimental Animals
At the University of Yaoundé, I and at the Institute of Medical Research and Medicinal Plants Studies, wistar strain mice with a weight varying between 100 and 150 g were obtained from the local animal house in Cameroon. At Ebony State University, Swiss albino mice weighing 18-25 g, were obtained from the local animal house in Nigeria. Mice were acclimatized to standard laboratory conditions for 7 days. Institutional animal ethical guidelines were strictly observed.
2.2. Plant Based Medicine and chemicals
Ngul be Tara is a 100% pure natural plant product manufactured by Reece International Research Consortium (Cameroon/Nigeria/USA), with plants grown in Cameroon, with equipment manufactured in the USA (Donated by Oregon Health Sciences University) and in Cameroon. All other chemicals used for the studies were of analytical grade and sourced from Ebonyi State Universit (Nigeria), from the Institute of Medical Research and Medicinal Plants Studies (Cameroon), and from the University of Yaoundé I, Faculty of Medicines and Biomedical Sciences University (Cameroon).
2.3. Acute toxicity
Acute toxicity was carried out in two universities: At the Ebonyi State University, Abakaliki College of Health Sciences at the Department of Pharmacology and Therapeutics (Nigeria) and at the University of Yaoundé I Faculty of Medicine and Biomedical Sciences, Department of Biochemistry and Physiological Sciences.
At Ebonyi State University, the LD50 of NBT powder extract was tested to determine its safety using Lorke (1983) method [16]. The study was carried out in two phases on Swiss albino mice weighing 18-25 g. The animals were grouped into three in the first phase, each group having three mice. They were administered NBT extract at doses of 10, 100 and 1000 mg/kg body weigh orally. The mice were observed for signs of toxicity and mortality for the first 4 hours and later 24 hours. In the second phase of the study, fresh set of three mice grouped into three with each having one mouse and orally administered 1600, 2900 and 5000 mg of the extract/kg body weight respectively. The mice were also observed for signs of toxicity and mortality for the first 4 hours and later 72 hours. Further monitoring was performed for seven days.
At the University of Yaounde I, Faculty of Medicine and Biomedical sciences, Department of Biochemistry and Physiology, acute toxicity studies where performed according to the OECD Guideline 420 basics: Acute Oral Toxicity - Fixed Dose Procedure was used. In this procedure, the initial dose level was selected on the basis of a sighting study as the dose expected to produce some signs of toxicity without causing severe toxic effects or mortality. Further groups of animals may be dosed at higher or lower fixed doses, depending on the presence or absence of signs of toxicity or mortality. This procedure continues until the dose causing evident toxicity or death is identified, or when no effects are seen at the highest dose or when deaths occur at the lowest dose. Twenty mice (10 males and 10 females) of the wistar strain with a weight varying between 100 and 150 g were divided into four groups of five animals each: Healthy female control (5 mice which received distilled water), Female test (5 mice who received the improved traditional medicine NBT), Healthy male control (5 mice who received distilled water), Male test (5 mice which received NBT). The test substance NBT was administered in a single dose of 5000 and 8000 mg/kg of body weight by gavage using a stomach tube. Animals were fasted prior to dosing. the following parameters over a period of 14 days were evaluated: LD50, general behavior of animals (aggressiveness, excessive cleaning, drowsiness, immobility, etc.); zootechnical criteria (food intake, water intake, weight change, weight gain); Macroscopic and microscopic observation of internal organs of animals after 14 days, in particular the heart, liver, lungs, kidneys, brain, adrenals and stomach; Histological analysis of target organs (liver, kidneys, lungs, heart); Biochemical analysis of toxicity markers (AST, ALT, gamma GT, Alkaline phosphatase, Direct bilirubin, Total bilirubin, Albumin, Total proteins, Creatinine, Urea and Uric acid). Information were collected on the hazardous properties that would allow NBT to be classified for acute toxicity according to the Globally Harmonized System of classification and labelling of chemicals.
From the result of an acute toxicity test, a conclusion can be made on the toxicity status of the test substance. Substances with LD50 below 5 mg/ kg are classified to be extremely toxic, 5–50 mg/kg - highly toxic, 50–500 mg/kg - moderately toxic, 500–5,000 mg/kg - slightly toxic, 5000-15,000 mg/kg - Practically non-toxic, and > 15,000 mg/kg - relatively harmless [17].
2.4. Histopathological studies
Histopathological studies of the liver, kidneys and lungs were carried out on control and experimental groups. Biopsies of each organ were fixed in 10% formalin, embedded in paraffin, sliced 4 µm thick and stained with hematoxylin and eosin (H&E), and then assessed for pathological changes [18].
2.5. Sub-acute toxicity
The sub-acute oral toxicity study was carried out following the OECD (2001) guidelines at the Institute of Medical Research and medicinal plants studies, Yaoundé-Cameroon. The albino mice were divided in to three groups of 6 per group and labeled as Group 1 – Control (H2O); Group 2 low dose administration (200 mg/kg) and Group 3 high dose administration (400 mg/kg). The H2O and extracts were administered to respective groups of mice daily for a period of 28 days by oral intubation. During the 28 days, mice were monitored weekly for water and food consumption, percentage of body weight gain. At the end of 28 days, mice were fasted overnight for 12 hours and blood was collected by retro orbital puncture using heparinized capillary tubes under anesthesia for plasma biochemical parameters to determine liver function (ALT, AST), Kidney function (Creatinine, urea), Lipids (Triglycerides, cholesterol) while whole blood was used for hematology (WBC, RBC, HB, HCT indices). Mice were then sacrificed by administering extra anesthesia. He liver, heart, kidney and spleen were isolated cleaned and weighed.
2.6. Statistical analysis
Data obtained were expressed as mean ± Standard deviation. Statistical analysis was performed using GraphPad Prism version 5.00 for windows in which one-way ANOVA was used followed by Waller Duncan multiple comparison tests at p < 0:05.
3. Results
3.1. Acute toxicity tests
The powder extract of NBT did not produce any lethality or visible signs of toxicity in mice up to the oral dose level of 5000 mg/kg body weight 72 hours after treatment. Further monitoring for seven days did not still yield mortality or visible toxic signs. Hence, the LD50 was ˃5000 mg/kg body weight.
3.1.1. Analysis of the weight gain of the study groups
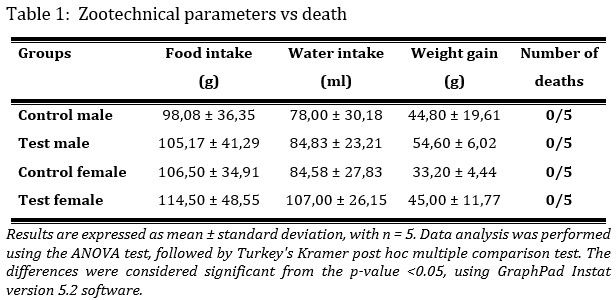
The kinetics of the weight development of the animals were observed. The animals of the different control and test groups show increasing weight gain over time. The comparative analysis of the weight gain shows a non-significant increase with a p-value > 0.05 of the groups treated with the extract compared to the groups that did not receive the extract. A weight gain of 32% is observed in the male control group, 36.35% in the male test group, 20.88% in the female control group and 31.16% for the female test group (Tab. 1). No death was recorded during the study period (Tab.1).
3.1.2. Analysis of food intake
The food intake during the study period was monitored. It reveals a non-significant increase with a p-value > 0.05 in food consumption in the test groups compared to the control groups (Tab. 1).
3.1.3. Water uptake analysis
Water intake was evaluated during the study period. It reveals a non-significant increase with a p-value > 0.05 in water consumption in test groups compared to control groups (Tab. 1). Table 1: Zootechnical parameters vs death
3.1.4. Analysis of the relative weight of the organs
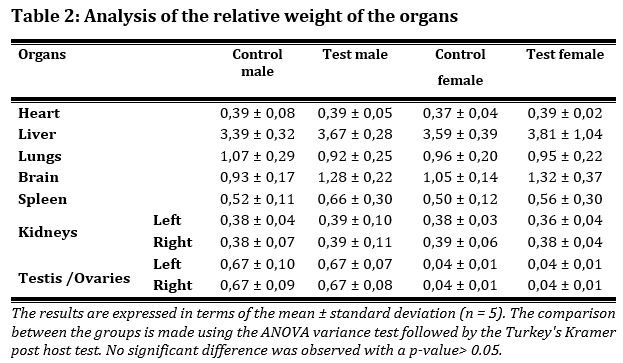
Analysis of relative organ weight did not show significant differences between groups of animals with a p-value > 0.05. Administration of ‘’Ngul be Tara’’ product does not affect the macroscopy of organs or the relative weight of the organs (Tab. 2).
3.1.5. Biochemical parameters
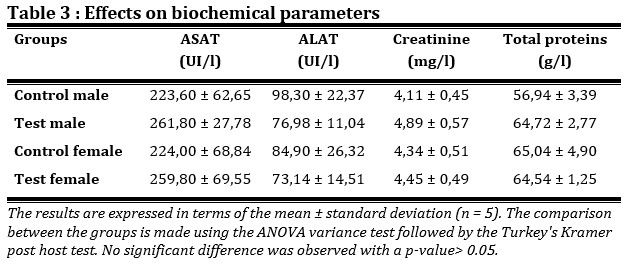
The effects of the product on biochemical parameters were closely monitored, a non-significant increase is observed with a p-value > 0.05 in the activity of the enzyme marker AST at high doses (8000 mg/kg of body weight) which is an enzyme specific to skeletal and cardiac muscles in the test groups compared to the control groups (Tab. 3). On the other hand, the administration of the plant-based product did not lead to an increase in the enzyme marker ALT, which is an enzyme specific to hepatic cells and kidney cells. Analysis indicates an absence of hepatic damage following administration of the product. Administration of the product results in a non-significant increase with a p-value > 0.05 in serum creatinine compared to the respective control groups at high doses (8000 mg/kg). Administration of the product did not result in a significant increase in serum protein levels with a p-value> 0.05 compared to healthy control groups. This reflects an absence of inflammatory reactions.
3.1.6. Histopathological analysis
Histopathological analysis of the liver
Histological analysis of the liver of different groups of animals did not show any major changes in the internal structure of the liver. A normal architecture of the centrilobular vein, sinusoids and even that of the hepatocytes in the control groups (Fig.1) were observed, and a slight obstruction of the central vein in the test groups that may suggest hepatic steatosis at very high doses ˃5000 mg/kg of body weight (8000 mg/kg of body weight). Overall, there is an absence of major pathological changes, even at high doses to which the animals are subjected. No pathological changes are observed at therapeutic doses.
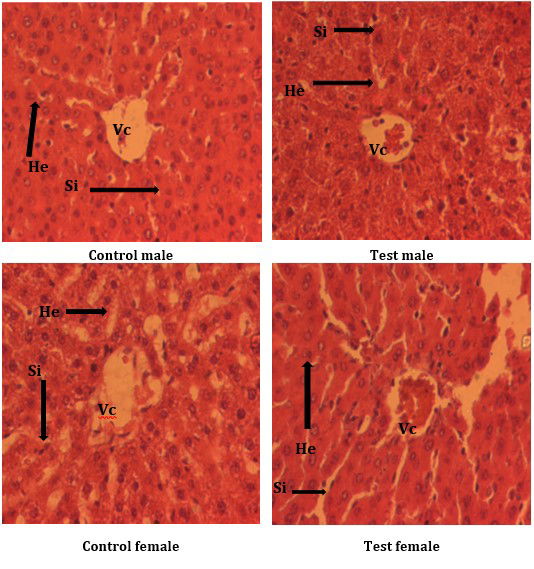
Figure 1: Histological sections of the liver of animals from different study groups. He = Hepatocyte; Vc = Central vein; Si = Sinusoid. He
Histological analysis of the kidney
The histological analysis of the kidney of the animals of the different study groups does not present any morphological alteration of the internal structure of the kidney (Fig. 2), whether at the level of the glomerulus, the Bowman’s capsule, the urinary space and the podocytes, whatever the doses used during the experiments.
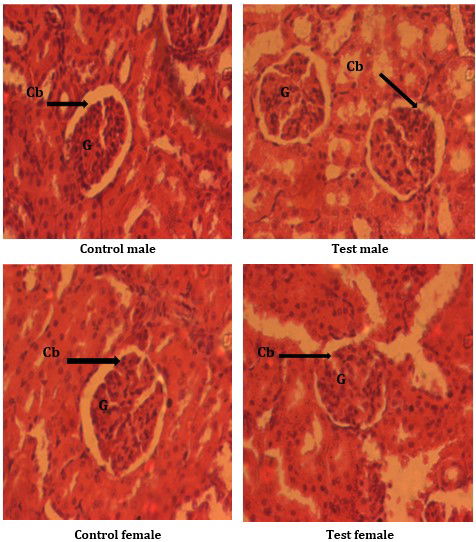
Figure 2 : Histological sections of kidney from animals from different study groups. Cb = Bowman’s capsule; G = Glomerulus.
3.2. Subacute toxicity tests
The effect of NBT on weight gain of experimental mice was monitored (Tab.4). A contend weight increase across the groups throughout the feeding period to the 4th week was observed. The weight gained in the Group 3 was significantly (p < 0.001) higher than that of the Group 1 and 2, which was maintained throughout the experiment. This difference was expected since from day zero the Group 3 Albino mice had higher weight than mice of the other groups
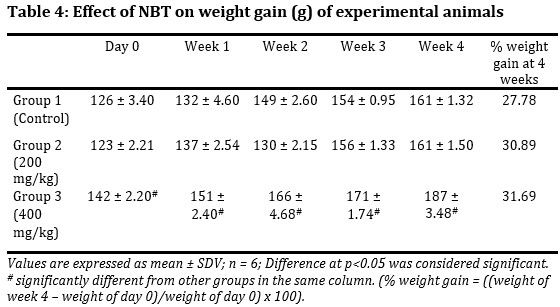
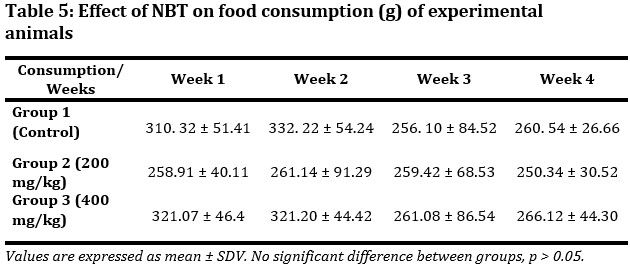
The effect of NBT on food consumption was evaluated (Tab. 5). Maximum food consumption for all three groups was obtained in week 2. However, with the exception of the week 2, the Group 3 animals had highest food consumption throughout. In all these no significant difference (p > 0.05) was observed between groups.
Table 5: Effect of NBT on food consumption (g) of experimental animals
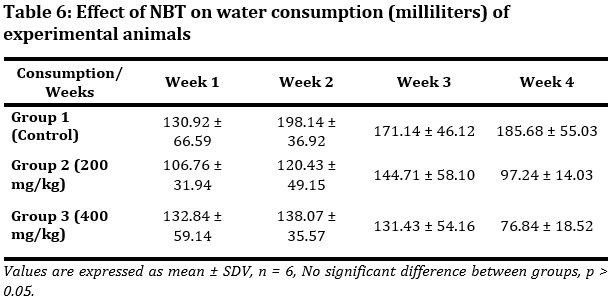
The effect of NBT on water consumption was monitored (Tab. 6). The Group 1 animals had the highest water consumption compared to Group 2 and 3. Meanwhile in the first 2 weeks Group 3 water consumption was higher than that of Group 2. And in the last 2 weeks Group 2 water consumption was higher than that of Group 3. In all these no significant difference (p > 0.05) was observed between groups.
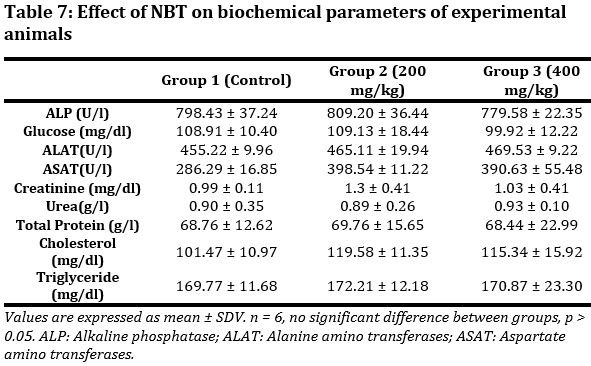
Biochemical parameters of mice treated with NBT were evaluated (Tab. 7). Though little variations in biochemical parameter was observed between groups, the differences were not significant was observed between groups for all parameters evaluated. All data obtained were within the physiological range.
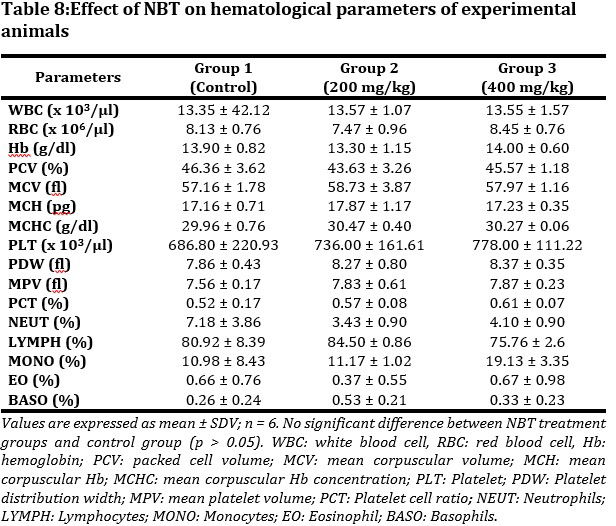
The effect of subacute administration of NBT on hematological parameters of experimental mice were observed (Tab. 8). Minimal though not significant variations between groups were observed for all parameters studied. The variations were within the physiological range.
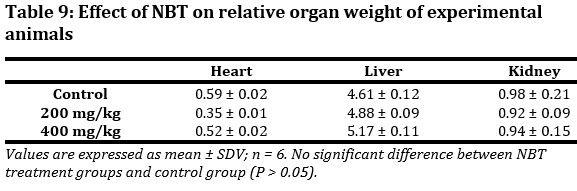
Daily administration of NBT did not cause any significant (p > 0.05) changes on selected organ weight (Tab. 9). Hence vital organs such as heart, liver and kidney were not negatively affected.
4. Discussion
Even though plants used in traditional medicines have generally been considered safe as a result of their long history of use in the treatment of diseases, pharmacological evaluation of medicinal plants is increasing amongst researchers worldwide. Research on the therapeutic potential of plants has surged over the years, with volumes of scientifically documented information showing considerable potential for medicinal plants to be used in the treatment of several diseases [19]. Currently there is no worldwide scientific consensus of a formulated drug judged effective for COVID-19 treatment. However, there is a consensus in the scientific community that natural products have been playing a dominant role in the discovery and development of drugs for the treatment of human diseases [20]. The majority of chemotherapeutic agents are based on natural products and this fact anticipates that new leads to fight pandemics may certainly emerge from the tropical plant sources, since biological chemodiversity, including in Africa, continues to be an important source of molecular templates in the search for drugs [21]. However, safety assessment of chemicals, pharmaceuticals, food ingredients and industrial products is very crucial prior to their approval for human uses.
In these studies, histopathology, acute and subacute toxicity testing were carried out to get more evidence on the safety of an African medicine NBT traditionally used for the prevention and treatment among others of respiratory infections including COVID-19. The acute administration of NBT led to the determination of an oral LD50 value ˃ 5000 mg/kg of body weight. Toxicity studies yielded comforting results. The said plant remedy NBT can be classified as practically non-toxic. Histological screening on vital organs of the body such as heart, spleen, liver, kidney, lungs and ovary showed no changes at doses lower than 5000 mg/kg of body weight. NBT did not affect the kinetics of weight growth in animals; did not cause the death of animals during the entire period of the study; caused a non-significant increase in weight gain, water intake and food intake; did not affect the macroscopy of internal organs of animals; did not cause a significant increase in AST; did not affect the internal structure of the kidneys or any other vital organ at doses up to 5000 mg/kg of body weight; seemed to cause at very high doses (8000mg /kg of body weight), a slight obstruction of the central vein of the liver which could produce hepatic steatosis; 8000 mg/kg of body weight is 200 to 400 times higher than the therapeutic recommended doses of 20-40 mg/kg of body weight , hence, prescribed doses should be respected.
Moreover, subacute oral administration of Ngul be Tara at doses 10 to 20 times higher than the recommended therapeutic dose showed that all tested parameters displayed no significant changes or were normal, did not produce signs of toxicity and hence may not be considered toxic in this case. All tested parameters were statistically judged similar in control and tested subjects namely: food and water consumption, percentage of body weight gain, organ (liver, kidney, heart) weight, liver function test (ALT, AST), kidney function tests (Creatinine, Urea), lipids (triglycerides, cholesterol) and blood glucose, hematology (WBC, RBC, HB, HCT indices). Similar results were found in previous studies; where oral acute toxicity of stem bark extract of NBT ingredients evaluated LD50 ˃5000 mg/kg of body weight [15], [22], [23], [24].
In conclusion, these studies, therefore have provided some scientific evidence for classifying NBT as practically non-toxic and its use in traditional, alternative or complementary medicine safe.
ACKNOWLEDGEMENTS
Financial support by the National Social Insurance Fund (CNPS) and its Director Mr Mekulu Mvondo Alain Olivier is highly appreciated. We thank Oregon Health Sciences University for equipment donation by the Former President Mr Jim Walker. We also thank the Institute for Medical Research and Studies of Medicinal Plants, IMPM, and its Director for participating in subacute toxicity studies. The participation of Reece International Research Consortium (RIRCO) researchers in the development of endogenous solutions and subsequent research is highly appreciated.
CONFLICT OF INTEREST
RIRCO is a not for profit community based organization promoting research and the development of improved science based natural indigenous medicines to help fight epidemics and global public health threats. RIRCO researchers provide assistance in both the development of improved traditional medicines and related research in resource limited settings.
REFERENCES
1. Nostro, A., Germanò, M.P., D'Angelo, V., Marino, A., and Cannatelli, M.A. (2000). ‘ Extraction methods and bioautography for evaluation of medicinal plant antimicrobial activity’. Letters in Applied Microbiology, 30(5), 379–385.
2. Mukharjee, P.K. (2002). ‘Quality Control of Herbal Drugs’, 1st Edition, Bussiness Horizon, Pharmaceutical Publishers, New Delhi, pp. 380-422.
3. Ekor, M. (2014). ‘The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety’. Front Pharmacol. Article ID: 24454289.
4. Dharmananda, S. (2017). ‘The Interactions of Herbs and Drugs’ [Internet]. Retrieved from URL: https://www.itmonline.org/arts/herbdrug.htm .
5. Kuete, V. (2014). ‘Toxicological Survey of African Medicinal Plants (1st edition)’. V, Kuete (Ed). (pp. 1-742). London: Elsevier Inc.
6. Ennell, C.W., Light, M.E., Sparg, S.G., Stafford, G.I., Van Staden, J. (2004). ‘Assessing African medicinal plants for efficacy and safety: Agricultural and storage practices’. Journal of Ethnopharmacology.95(2-3), 113-121.
7. Merlin, L., Mensah, K., Gustav, K., Arnold, D.F., Caleb, F., Alexander, K.A., and Rita, A.D. (2019). ‘Toxicity and Safety Implications of Herbal Medicines Used in Africa’. Retrieved from URL: https://www.intechopen.com/chapters/58270 Last accession date: 04/03/2022. DOI: 10.5772/intechopen.72437.
8. Gao, S., Ying, M., Yang, F., Zhang, J., and Yu, C. (2020). ‘Zhang Boli: Traditional Chinese Medicine plays a Role in the Prevention and Treatment on Novel Coronavirus pneumonia’. Open access online-first publ res pap COVID-19. Geneva, Switzerland: WHO. 121–124. Retrieved from URL: http://en.gzbd.cnki.net/ GZBT/brief/Default.aspx.
0. Ekor, M. (2013). ‘The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety’. Front. Pharmacol. Vol.4 DOI: 10.3389/fphar.2013.00177.
10. Chalut, D.S. (1999). ‘Toxicological risks of herbal remedies’. Paediatr. Child Health. 4: 536–538.
11. Adote, A.J., Adukpo, G.E., Yaw, G., Armah, O.B.F. (2012). ‘A Review of the Ethnobotany and Pharmacological Importance of Alstonia boonei De Wild (Apocynaceae)’. ISRN pharmacology. Article ID: 587160. 10.5402/2012/587160.
12. Amole, O.O., and Ilori O.O. (2010). ‘Antimicrobial activity of the aqueous Andethanolic extracts of the stem bark of Alstonia boonei’. International Journal of Phytopharmacology, 1(2), 119-123.
13. Tan, P.V., Boda, M., Enow-Orock, G., Etoa, F., Bitolog, P. (2007). ‘Acute and sub-acute toxicity profile of the aqueous stem bark extract of Enantia chlorantha oliver (annonaceae) in laboratory animals’. Pharmacologyonline 1: 304-313.
14. Noreen, O.K.M., Aristide, T., Alain, S., Marlaine, M.B.M., Raissa, R.A.S., Sylvin, O., Alfred, S.T. (2016). ‘Preliminary studies of acute and sub-chronic toxicity of the aqueous extract of Guibourtia tessmannii (Harms) J. Leonard stem barks (Caesalpiniaceae) in mice and rats’. Asian Pacific Journal of Tropical Biomedicine, vol. 6, no. 6. doi:10.1016/j.apjtb.2016.04.001
15. Kwan, Y.P., Ibrahim, D., Yeng, C. (2013). ‘Subramaniam Sreeramanan, Sreenivasan Sasidharan, Acute and Subchronic Toxicity Study of Euphorbia hirta L. Methanol Extract in Rats’. BioMed Research International, 2013: 14, Article ID 182064. Retrieved from URL: https://doi.org/10.1155/2013/182064.
16. Lorke, D. (1983). ‘A new approach to toxicity testing’. Archives of of Toxicol, 54:275-287.
17. Loomis, T.A., Hayes, A.W. (1996). Loomis’s essentials of toxicology (Fourth 4 Edition). pp. 208–245. California: Academic press
18. Kasote, D.M., Badhe, Y.S., Zanwar, A.A., Hegde, M.V., and Deshmukh, K.K. (2012). ‘Hepatoprotective potential of ether insoluble phenolic components of n-butanol fraction (EPC-BF) of flaxseed against CCl(4) induced liver damage in rats’. Journal of Pharmacy and Bioallied Sciences 4: 231-235. Retrieved from URL: http://dx.doi.org/10.4103/0975-7406.99064.
19. Benzie, I.F., Wachtel-Galor, S. (2011). Herbal Medicine: Biomolecular and Clinical Aspects (2nd Edition). CRC Press-Taylor & Francis; Boca Raton, FL, USA.
20. Newman, D.J., Cragg, G.M., and Snader, K.M. (2003). ‘Natural products as sources of new drugs over the period 1981-2002’. J. Nat. Prod. 66: 1022-1037. 2.
21. Portet, B., Fabre, N., Roumy, V., Gornitzka, H., and Bourdy, G. (2007). ‘Activity-guided isolation of antiplasmodial dihydrochalcones and flavanones from Piperhostmannianum var.berbicense’. Phytochemistry, 68: 1312-1320.
22. Iyiola, O.A., Tijani, A.Y., and Lateef, K.M. (2011). ‘Antimalarial Activity of Ethanolic Stem Bark Extract of Alstonia boonei in Mice’. Asian Journal of Biological Sciences, 4: 235-243. DOI: 10.3923/ajbs.2011.235.243.
23. Ajani, E.O., Ibrahim, L.B. (2020). ‘Toxicological evaluations of combined administration of ethanolic stem bark extract of Enantia chlorantha and lisinopril in experimental type 2 diabetes’. Clin Phytosci6, 29. Retrieved from URL: https://doi.org/10.1186/s40816-020-00174-z.
24. Rajeh, M.A., Kwan, Y.P., Zakaria, Z., Latha, L.Y., Jothy, S.L., and Sasidharan, S. (2012). ‘Acute toxicity impacts of Euphorbia hirtaL extract on behavior, organs body weight index and histopathology of organs of the mice and Artemia salina’. Pharmacognosy research, 4(3), 170–177. Retrieved from URL: https://doi.org/10.4103/0974-8490.99085.
AUTHOR INFORMATION
1 Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, Ebonyi State University, Abakaliki, Nigeria
2 Institute of Medical Research and Medicinal Plants Studies, Center for Research on Medicinal Plants and Traditional Medicine, Ministry of Scientific Research and Innovation, Cameroon
3 Department of Public Health, Faculty of Allied Health Sciences, King David University of Medical Sciences, Uburu. Ebonyi State, Nigeria
4 Department of Biochemistry, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
5 University Institute Joseph Ndi-Samba, Yaounde Cameroon
6 Chantal Biya International Reference Centre for Research on Prevention and Management of HIV/AIDS (CIRCB), Yaoundé, Cameroon