African plant remedies’ mix containing Ngul be Tara, Netko, Binther & Immunoboost treats COVID-19 patients and blocks its transmission: a comparative observational study on 552 patients
Cite: Malongte, P., Oben, O.S., Lepafo-Moffo, B.R., Ngah-Ngah, S., Handy-Dissack, S., Bami-Feumi, F.J., Ngono-Atangana, C.E., Ngoa-Nnanga, C.C., Tiburce Nyiama, T., Nwaze, E.O. and Matsezou, J.(2022). 'African plant remedies’ mix containing Ngul be Tara, Netko, Binther & Immunoboost treats COVID-19 patients and blocks its transmission: a comparative observational study on 552 patients.' The Int. J. Holist. Sci. Innov. Dev., 11(3), pp. 47–88.
African plant remedies’ mix containing Ngul be Tara, Netko, Binther & Immunoboost treats COVID-19 patients and blocks its transmission: a comparative observational study on 552 patients
Pierre Malongte1,2, Sammy O. Oben3, Baurel R. Lepafo-Moffo4, Symplice Ngah-Ngah4, Sylvie Handy-Dissack5, Franck J. Bami-Feumi6,7, Chantal E. Ngono-Atangana1, Corinne C. Ngoa-Nnanga8, Tiburce Nyiama1, Eric O. Nwaze9 and Jacqueline Matsezou1
Corresponding author: Baurel Lepafo-Moffo, lepafobaurel@yahoo.com - Accepted in the revised format: October 2022 - Published: November 2022
Abstract
Almost 3 years since the start of the SARS-CoV-2 pandemic and over 6 million deaths so far, the world continues to be on high COVID-19 alert. Despite the low death rate recorded in the majority of African countries, very little attention has been given to African plant remedies full of healing compounds such as Polyphenols, Flavonoids, Tannins, Coumarins, Terpenoids and others. In developing countries, traditional medicine constitutes a major part of health care because of its local availability and affordability. Its efficacy in clinical settings is poorly documented with regards to many illnesses it addresses, including COVID-19. As of writing, there is no worldwide validated medicine to address that pandemic disease, except for some promising or repurposed drugs that have received emergency authorization such as chloroquine or hydroxycholoquine. In this research a comparative observational study was conducted, on 552 patients and their close-contacts randomly selected out of 5000 treated people, to determine whether COVID-19 Improved African Traditional Medicines (MTAs) can prevent or cure COVID-19. To address this question, the outcome of patients treated with 2 protocols made of Ngul be Tara alone 1 teaspoon 3 times a day for 10 days, or within a kit associated with Netko 1 table spoon twice a day, Binther 1 teaspoon twice a day and Immunoboost one teaspoon once a day for 6 days, was compared to that of those treated with Chloroquine 200 mg 3 times a day. We also assessed Ngul be tara efficacy as a post exposure prophylactic agent; in doing so, the outcome of close-contacts of positive tested patients who received a preventive treatment with Ngul be Tara 1 teaspoon (50 mg/kg of body weight) twice a day for 10 days was measured. We obtained a cure rate of 100 % with Ngul be Tara alone or Ngul be Tara/Netko/Binther (Anti-covid-19 Kit), a cure rate of 79.4% with chloroquine, defined as a positive test turned negative after a 6 to10 day treatment combined with the relief of disease symptoms and hospital discharge. Following statistical evaluation at the 5% threshold, it is concluded that the cure rates of patients on Chloroquine vs Anti-COVID-19 Kit (p-value = 0.00008989 <0.05), Chloroquine vs Ngul be Tara alone (p-value = 0,004308<0.05) are significantly different. In terms of the duration of symptoms, in patients treated with chloroquine, symptoms significantly lasted longer with a duration period of 10.03 ± 2.87 days, compared to that observed in patients treated with improved traditional medicines: with the Anti-COVID-19 kit, symptoms’ duration was evaluated at 6.39 ± 2.6 days, and with Ngul be Tara alone 4.13 ± 2.54 days. All close contacts who received prophylactic doses of improved traditional medicines were free from SARS-COV-2 infection for over a one-month observation period. Results are statistically significant and indicate that MTAs can address from asymptomatic to severe cases of SARS-COV-2 infections. MTAs evaluated treat and prevent Coronaviruses’ diseases.
Key words: African traditional medicine, Ngul be Tara, Netko, Binther, Immunoboost, Chloroquine, clinical efficacy, prophylaxis, coronavirus, COVID-19.
Introduction
Coronaviruses are enveloped, positive-stranded RNA viruses that negatively influence the respiratory, intestinal, liver, nervous and other systems of animals and humans [1-2]. The current WHO declared pandemic of Coronavirus disease 2019 (COVID-19), is caused by the Severe Acute Respiratory Syndrome Coronavirus two or SARS-COV-2 [3]. It is a novel betaCOV belonging to the same subgenus as the Severe Acute Respiratory Syndrome Coronavirus (SARS-COV) and the Middle East Respiratory Syndrome Coronavirus (MERS-COV), which have been previously implicated in SARS-COV and MERS-COV epidemics with mortality rates up to 10% and 35%, respectively. About 8096 cases and 774 deaths were reported worldwide with the SARS-COV infection during year 2002; 2229 cases and 791 deaths were reported for the MERS-COV that emerged during 2012 [4]. Like other RNA viruses, SARS-COV-2, while adapting to its new human hosts, is prone to the development of several mutations over time, resulting in mutant variants that may have different characteristics than its ancestral strains [5]. Several variants of SARS-COV-2 have been described, among which a few are considered variants of concern (VOCs) by WHO. Based on their epidemiological update, as of December 2021, five SARS-COV-2 VOCs had been identified since the beginning of the pandemic. Despite the performance and increasing availability of newly developed vaccines, these emerging VOCs, which appear to evade immune responses triggered by vaccines, represent a significant concern to immunization strategies [6].
The origin of the virus is unknown, the emergence of the disease was seemingly first noticed in Wuhan city, China, East Asia [7]. According to a recent Chinese study, about 80% of patients have mild illness and the overall case fatality rate is about 2.3% but reaches 8.0% in patients aged 70 to 79 years and 14.8% in those aged 80 and over [8]. It is believed that there is likely to be a large number of asymptomatic carriers in the population, and the mortality rate is probably overestimated. However, this disease remains highly contagious. Indeed, analyzes of the transmission of COVID-19 in Shenzhen, China, have shown secondary infection rates at home and in close contact of 15% and 10%, respectively [9]. Other studies reported a COVID-19 secondary attack rate of about 13% among household contact [10]. A study in Spain conducted on 466 household contacts from 227 index cases reported a secondary attack rate of 58.2% during the Delta-dominant period and 80.9% during the Omicron-dominant period [11].
In response to the world panic, social distancing, hand washing, alcoholic disinfectants or hand sanitizers, isolation/quarantine, travel restrictions, wearing of face mask, community containments and partial or total lockdown are non-pharmaceutical preventive measures advised. Alongside several experimental vaccines having benefited from emergency authorization in an effort to halt severe cases, hospitalization and deaths [12].
Despite mass vaccination, globally, as of 8 December 2021, there had been 266,504,411 confirmed cases of COVID-19, and 5,268,849 deaths, reported to WHO. As of 7 December 2021, a total of 8,026,232,437 vaccine doses had been administered [13]. However, Evidence is emerging on immune escape capabilities of the variants [14-16]. Despite the unprecedented speed of vaccine development for the prevention of COVID-19 and robust global mass vaccination efforts, the world continues to be on high COVID-19 alert. Hence the importance of developing additional efficient prophylactic agents and treatments. With an emphasis on post-exposure prophylaxis (PEP) agents which has the theoretical advantage of preserving stocks of drugs and limiting emergence of antimicrobial resistance.
In terms of treatment, a variety of conventional therapeutic options are available mostly in industrialized country and that include antiviral therapies such as remdesivir, Lopinavir/ritonavir [17], nirmatrelvir, molnupiravir, ivermectin [18], anti-SARS-COV-2 Neutralizing Antibody Products such as convalescent plasma therapy (CPT), casirivimab/imdevimab, anti-inflammatory drugs (e.g., dexamethasone), immunomodulators agents (e.g., baricitinib, tocilizumab, bamlanivimab/etesevimab); most are repurposed or available under Emergency Use Authorizations or being evaluated in the management of COVID-19 [19]. In 2017, a study on remdesivir proved its efficacy against MERS-COV, SARS-COV, and bat-COV, which indicates its broad-spectrum anti-human-COV activity [20].
The drugs mentioned above and the CPT confer some relief to the pandemic situation. Still, the exorbitant therapy, limited availability, unprecedented adverse effects of some of the drugs and ethical concerns regarding CPT make them difficult to implement worldwide. Moreover, the situation can be exacerbated by the mutation of the virus leading to drug-resistant mutants, which would render the anti-viral drugs useless since most of them target unique specific viral proteins [21-24]. It’s important to keep developing potential affordable remedies. Extensive studies have shed some light on natural products' mechanism of action and the possible targets like viral entry, replication, assembly, release, and virus–host specific interactions [25]. Central to designing effective medicines is the understanding of the said interactions and subsequent disease symptoms.
It has been reported that the onset of symptoms occurs on average 5–6 days after exposure, and normally, those with mild symptoms recover within 2 weeks; however, in severe cases, the recovery may extend up to 6 weeks [26]. In some patients, regardless of the disease severity, the symptoms may persist or recur for weeks or months following initial recovery [27]. The clinical data presentations in COVID-19 patients have revealed that of the total cases, about 80% either are asymptomatic or have mild symptoms, while around 14% develop severe symptoms, such as pneumonia; around 5% develop critical symptoms, such as septic shock, respiratory failure, or multiorgan failure; and about 2% of the patients die of the disease [28]. Fatality is comparatively much higher in the aged and persons with comorbidities [29-30].
Comorbidities have been the most significant host factors contributing to COVID-19 severity. The most frequent contributors to COVID-19 have been respectively cardiovascular diseases, diabetes, chronic respiratory disease, hypertension, and obesity [31].The widespread expression of SARS-COV-2 host cell entry factors across human tissue types such as angiotensin-converting enzyme-2 (ACE2) and neuropilin-1 (NRP1), and the dysregulation of the renin–angiotensin–aldosterone system (RAAS) that regulates physiological hemodynamic balance involving all major organs namely liver, lung, heart, and kidney, have been characteristic features in COVID-19 [32] leading to disease severity. Moreover, a hyperactive innate immune response accompanied by delayed or suppressed adaptive immunity seem to be key factors behind systemic manifestations and poor clinical outcomes in the patients. The inclusion of Furin Cleavage site in the spike protein sequence may also be a reason for the increased virulence of SARS-COV-2 [33]. Additionally, dampening of the early IFN response, subsequent cytokine storm and suppressed/delayed adaptive immune response marked by intense lymphocytopenia and suboptimum synthesis of immunoglobulins are the prominent immunological features, which seem to drive the severity of the disease [33]. Moreover, it is believed that gut microbiome has a significant role in disease severity and patient outcomes in COVID-19, as it builds up the local mucosal immunity against viral invasions [34]. Studies indicated that patients with COVID-19 have a different microbiome compared with controls, it is enriched with the opportunistic pathogens, and depletion of the beneficial commensals showed a correlation with severity of the symptoms [35]. They also indicated that the composition of the gut microbiome may influence SARS-CoV-2-induced production of inflammatory cytokines and consequently in the onset of a cytokine storm [36,37].
Every unveiled feature or step of the disease evolution may represent a potential target for drugs’ active ingredients. Indeed, the clinical course of the COVID-19 illness occurs in 2 phases, an early phase when SARS-CoV-2 replication is greatest before or soon after the onset of symptoms. Antiviral medications and antibody-based treatments are likely to be more effective during this stage of viral replication. The later phase of the illness is driven by a hyperinflammatory state induced by the release of cytokines and the coagulation system’s activation that causes a prothrombotic state. Anti-inflammatory drugs such as corticosteroids, immunomodulating therapies, or a combination of these therapies may help combat this hyperinflammatory state more than antiviral therapies [38].
During the disease course, it has been shown that SARS-CoV-2 infection also causes mitochondrial dysfunction in bronchial epithelial cells and macrophages, and therefore, the mitochondria becomes dysfunctional for regulating intracytoplasmic Fe metabolism with an increase in reactive oxygen species (ROS), and the process culminates in what may be referred to as ferroptosis [39]. Excess Fe can be tolerated to a limited extent, as is the case of silent hypoxia. Ferroptosis associated with multiorgan oxidative stress can precipitate the cytokine storm in later stages of the disease, for instance, in critically ill patients. The laboratory examination of critically ill patients indicates low hemoglobin and high ferritin levels in non-surviving patients [40]. It is known that oxidative stress and cellular damage stimulated by iron is a source of Fenton reaction and its product hydroxyl radical (•OH), the most reactive ROS with t1/2 of 10−9 s [41]. Thus justifying the administration of antioxidant therapies.
Hence the use of epigenetic mechanisms or potential ROS scavengers in conventional therapies to alleviate COVID-19 symptoms such as: corticosteroids, heparin, tissue plasminogen activator, stilbene, ebelsen, metformin, tocilizumab, aculizumab, interferon β and γ, interleukinin 1 inhibitors, mesenchymal stem cells, nitric oxide, cannabidiol, N-acetylcysteine, calcifediol, vitamin C, D and carotenoids [42-49]. Il has been suggested that potential therapies in hemoglobin dysfunction and COVID-19 pathophysiology can be found in natural compounds with antioxidant properties found in plants; for example, curcumin, quercetin, phloretin, berberine, sulforaphone, carotenoids and many others which show a preventive potential towards SARS-COV-2 infection [50].
While most of the affected countries in Europe and America are relying mostly on conventional drugs, South East Asia, and in particular China where the COVID-19 pandemic seems to have originated, has adequate documentation of successful outcomes following the integration of Traditional Chinese Medicine (TCM) with conventional medicines in COVID-19 management [51]. China and neighboring Asian countries practice an age-long traditional medicine system that has been favorably integrated with the western medicine; the TCM-western system of healthcare was therefore adopted to combat the earlier outbreak of SARS-COV in Guangdong, China in 2002 leading to the reported defeat of the epidemic [52]. Poly-herbal formulations containing many indigenous plants use for prophylactic purposes among health workers against SARS-COV infection or as adjuvants is documented [53]. Based on historical records and human evidence of SARS and H1N1 influenza prevention, Chinese herbal formulas have been found to be an alternative approach for prevention of COVID-19 in high-risk population. Results from 3 studies using TCM for prevention of SARS and 4 studies for H1N1 influenza showed that none of the participants who took TCM contracted SARS in the 3 studies [54]. Following the reported success with the use of herbal adjuvants during the previous outbreaks of viral infections in China, the outbreak of SARS-COV-2 received an authorization of integral Traditional Chinese–Western medicines to treat COVID-19 [51].
In Africa, the use of phytomedicines also referred to as herbal medicine or phytotherapy is well embraced in different African territories where 80–90% of its rural populations rely mainly on plant-based traditional medicines for primary healthcare. [55,56]. There has been a heavy use of phytomedicines in Africa following the outbreak of COVID-19 pandemic.
To date, there is no worldwide validated treatment, a situation which has been compounded by lack of authorized medicines that are judged effective, affordable and accessible to all populations. Coincidentally, available evidence from Africa shows that the African continent is the last to be hit by the viral pandemic and the least affected continent so far after Oceania with around less than 4% of the world COVID-19 death toll [57,58]. Even though WHO studies reported that over 2/3 of the African 1.4 billion population (around 16.72% of the total world population) [59] is believed to have been exposed to SARS-COV-2 [60]. While several factors may be attributable to this seeming positive trend, the dependence on the African medicinal plants for COVID-19 management may not be ruled out. As a malaria endemic region, Sub-Saharan Africa often co-administer herbal remedies alone or combined with conventional drugs; and many of these plant-based medicines have been informally repurposed by various users for COVID-19 prevention and symptomatic management as home remedies. For example, several African plants are rich in quinolines which are heterocyclic molecules composed of fused benzene and pyridine rings. The quinolines and their derivatives have shown a wide range of biological activities, including antiproliferative [61], antiviral [62], antibacterial [63], antifungal [64], anti-inflammatory [65], and antiparasitic [66]. Members of the quinoline family, such as chloroquine and hydroxychloroquine, have shown antiviral activity against several viruses, such as coronaviruses, human immunodeficiency virus [67], respiratory syncytial virus [68,69] and West Nile virus [70,71]. Concerning Flavivirus, quinoline derivatives have proved active against the Hepatitis C virus [72], Japanese Encephalitis virus [73], Zika virus [74], and dengue virus [75].
Unlike the Traditional Chinese Medicine, there is a scarcity of published studies on the impact and efficacy of the widely used traditional African Medicine adopted for the prevention, management and treatment of COVID-19. However, the State of Cameroon has registered 9 improved category II traditional medicines that are used along with several promising category I improved traditional medicines to complement conventional therapies. This study is therefore aimed at providing more insights into the efficacy of African medicinal plants in clinical settings and their therapeutic potentials in the prevention and management of COVID-19.
Materials and methods
Type of study
The present observational analytic study is the follow-up of a multicenter emergency intervention. A national validated reference treatment is used as a control in order to assess the efficacy and tolerability of researchers' improved traditional medicines (MTAs) in Cameroon. This observational study consisted of collecting data on the treatment administered to people who had tested positive to COVID-19, their close contact, and who had used MTAs for treatment or prophylaxis or the validated conventional national protocol based on chloroquine. This is an analytic descriptive cross-sectional study with retrospective data collection. Data on results and unexpected effects were collected from individuals and health workers. This study combines quantitative and qualitative research techniques.
Data collection techniques
Four main techniques were used to collect data including: 1) literature review 2) desktop review 3) a random selection survey using a tool administered through semi-structured interviews with individuals who had used MTAs in confinement places, 4) patient record review by systematic sampling of in-patients who tested positive to COVID-19 and were monitored by health personnel from 2 hospitals who have chosen the MTAs or chloroquine.
Survey frame
The survey consisted of people exposed to inter-community contamination of COVID-19 in the city of Yaoundé, meaning: people tested positive for COVID-19; people who have been exposed to the virus, tested or not, and who have used improved traditional medicines (MTAs). The observation unit was an individual, female or male with a positive COVID-19 test, laboratory diagnosed and treated with MTAs, a patient having a clinical diagnosis showing the presence of COVID-19 type lung lesions (glass powder appearance) and symptoms treated with MTAs; a person who requested Ngul Be Tara for prophylaxis, and finally a person who was treated with the combination Chloroquine, Azythromycin and zinc (national protocol). The sampling frame was constituted by patients who used African Traditional Medicines or chloroquine for prophylaxis or curative treatment, along with their close contacts, including family members and co-workers sharing offices, worship places or other very closed environment. The 5000 people treated between May and August 2020 mainly in the city of Yaoundé, by care givers from 2 health facilities, in confinement sites and their homes, and whose family voluntarily requested treatment, taking into account the various people in close contact with them, represented our survey frame.
Sample size
Calculation of the sample size was based on the scientific method determining the minimum size necessary to achieve a level of precision of the desired results [76]. The precision calculation was based on a few control indicators in terms of proportion or average: From the 5000 observed population, the minimum sample size necessary to achieve an accuracy of d = 5% is n = 384. This means 384 or more surveys are needed to have a confidence level of 95% that the real value is within ±5% of the surveyed value.
The size of proportions to be compared plays a crucial role in calculating the power of a test, that is, its ability to reject the null hypothesis. When the groups have unequal size, the expression for calculating the size of the first group nA with the size of the second group set at 39 takes the following form:
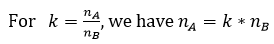
The difference in numbers may be made necessary, in particular for ethical reasons. There is a gain in power, at least up to a value of k inferior or equal to 5, however, there is a significant increase in methodological constraints.
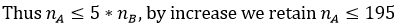
Thus, a patient sample was selected in such a way that the ratio of the different treatment groups will be comparable (ratio less than 5). One category to be compared to another cannot be more than 5 times greater than the other.
Inclusion criteria
Inclusion criteria included: 1) Person tested positive for COVID-19 at nationally registered health facilities, diagnosed in the laboratory and treated with MTAs and their close contacts; 2) Person tested positive for COVID-19 at nationally registered health facilities, diagnosed in the laboratory and treated with chloroquine; Person 2 years of age or older.
Exclusion criteria
People with the following characteristics were excluded: 1) Person who had not been treated with MTAs or chloroquine; 2) Person less than 2 years of age; 3) Pregnant women.
Sample constitution
The sample constitution method retained was simple random sampling for outpatients. Inpatients treated by the 2 institutions’ caregivers were systematically selected (Hadassah and SOS AMY). Once the minimum sample size of 384 patients was retained, and the proportions’ comparison principle ascertained up to the size of 195 per group, operations to clear the database were carried out and essentially focused on quantitative questions such as age, durations, etc. All databases were grouped into one to allow comparison. With regard to the quantitative variables, the imputation was made through the mean according to the groups. For age, the missing values were replaced by the average age by sex. For the duration of the symptoms, the imputation was made by the average of the durations according to the treatment administered. For multiple-response variables such as symptoms and comorbidities, the different responses were dichotomized so as to bring out the frequencies.
Data collection tools
Two main types of data collection tools were designed. One data collection form for inpatients and one for outpatients and interview guides for data collectors (health professionals). A pretest was performed and forms were reviewed before launching data collection. Health workers were trained for efficient data collection.
Collected Information
Information collected on inpatients were: 1) Socio-demographic characteristics : coded name, age, gender, marital status, contact address and phone number for follow up, number of children ; 2) Clinical situation of the patient (perceived): - clinical signs on admission with COVID-19 list of symptoms to choose from ( fever, cough, fatigue, aches, headache, diarrhea, dyspnea and other signs); Exposure through: travel, work , Contact with infected people or Contact with people at risk: 3) Diagnostic data:- Clinical diagnosis, based on the clinic (minor and major symptoms diagnosed by the health worker); Imaging : X-Ray Radiography, thoracic Scanner; Laboratory diagnosis: RDT (IgM, IgG, Ag); PCR; 4) Treatment received & doses; 5) Direct daily observations from Day 1 to day 7: symptoms, side & unexpected effects monitoring; 5) Duration of treatment; 6) Duration of hospital stay; 7) Evaluation after one week post treatment; 8) Follow-up 4 weeks post treatment; 9) Satisfaction level. Information collected on outpatients and patients in confinement places were: 1) Socio-demographic characteristics : name coded, age, gender, marital status, profession, salary, contact address and phone number for follow up, age, number of children ; 2) Diagnostic data:- Clinical diagnosis , based on the clinic; Laboratory diagnosis: RDT (IgM, IgG, Ag); PCR or other; 3) Clinical situation of the patient or contact: - 4) Comorbidities or preexisting diseases; 5) Clinical symptoms ; 6) Duration of symptoms following treatment administration; 7) Medicines used including presentation; 8) Treatment doses; 6) The effectivity of the control test; 7) The result of the control test ; 8) Resistance to treatment; 9) Unexpected or side effects;10) Satisfaction level, 11) Comments: 12) Follow-up 4 weeks post treatment.
Duration of data collection: data were collected during the month of September 2020 for 30 days at the hospitals and in confinement sites. Additional information for the final follow up was obtained through phone calls.
Data Management and Statistical Analysis
Data were collected, codified and analyzed using three software namely: Excel (v16.0), SPSS (v21.0) and R (v4.0). Data were codified to ensure patients and other participants’ confidentiality.
Principles of comparison tests: the work carried out did not relate to the entire population and the constitution of the samples was made with little information on the said population (overall exposed population), to generalize the statistical results from the samples to the population concerned we used a set of statistical tests. In addition, carrying out tests requires the satisfaction of a certain number of conditions which depend on the nature of the data (nominal or continuous) and the distribution of the samples (normality and homoscedasticity). These last sine-qua-non conditions for performing parametric tests required us to use non-parametric tests when at least one of these conditions was not met. The scheme [77] below presents the logical continuation in the choice of the type of statistical tests appropriate according to the situation.
Thus, beforehand, tests of normality and homoscedasticity of the distribution were carried out before comparison tests. The comparison was performed two by two: on the one hand between Chloroquine and the anti -Covid-19 Kit protocols; and on the other hand between Chloroquine and Ngul be Tara. With regards to nominal variables, the verification of normality and homoscedasticity of distributions was no longer necessary and in this case, we used the test of comparison of proportions, including that of Chi-2. In all of the tests used, the significance was concluded based on the p-value. Thus, for a test formulated by: H0∶ Null Hypothesis and H1∶Alternative Hypothesis, the null hypothesis is credible if p-value is greater than a threshold set in our study at 5%.
Ethical consideration
No interventions were performed during the study and retrospective data were collected. The following principles were applied: respect for participants, goodwill and justice (Belmont, Beauchamp and Childress). The respect for people refers to autonomy, free and informed consent and confidentiality, and includes the protection of persons with loss of autonomy; in this study, respondents were not simply used as a way to achieve the research objective. The goodwill demonstrated consisted in minimizing risks and maximizing benefits, including psychological and social risks. Justice referred to the equitable distribution of the risks and benefits associated with participation in the research. All participants were treated fairly and had access to available information, methods and research results.
Informed consent: before any research or questioning, the consent of the participant was obtained; possible risks of confidentiality were analyzed with them. Only one mature and competent person gave informed consent at a time. For students, informed consent was obtained from parents. Consent was obtained by reading the participant consent form, then having the participant sign the document or verbally declare their consent (depending on their choice). All participants were given the opportunity to decline to participate based on an understanding of what is asked to do and fully understood that there are no real implied or negative consequences for refusal. The informed consent form was read before any research; and describes the purpose of the research, indicates the name of the sponsor, explain the risks and benefits, indicates that the research is voluntary and that the person can withdraw at any time, without impact or consequences. An information notice was also attached to it, with contact information of people to contact if necessary.
Results
LITERATURE REVIEW DATA
Since a first case was confirmed on the 5th of March 2020, COVID-19 had quickly spread beyond the two main cities Douala and Yaoundé, making Cameroon one of the main COVID-19 hot spots in West and Central Africa [78]. In the country, during the period targeted for data collection, total recorded cases varied from 1832 on May first to 19142 on August 30th 2020 [79]. Five thousand cases (an equivalence of over ¼ of Cameroon officially declared cases within the 3-month period) were treated or monitored by health care workers from SOS AMY and Hadassah. From that population, 285 patients were selected for data collection, along with their 267 close contacts who had taken a prophylactic treatment. In the entire country, currently infected people varied from 837 on May 1st to 1080 on August 31, 2020 [79]. Meanwhile total recorded death fluctuated from 61, as of May 1st to 411 on August 31st, 2020. As of September 9, 2020 according to the Ministry of public health, there was 194 countries hit by the pandemic worldwide with 27 486 960 confirmed cases and 894 983 deaths (3,3% lethality rate); during the same period in Africa, 54 countries were hit by COVID-19, with 1 315 073 confirmed cases and 31 725 deaths (2.4 % lethality rate); in Cameroun, 20 009 confirmed cases were recorded with 415 deaths (2,1% lethality rate) [80]. During the same period, Cameroon was the 9th country with the highest number of confirmed cases in Africa after, respectively, South Africa, Egypt, Morocco, Ethiopia, Nigeria, Algeria, Ghana, Kenya [80]. The country has 10 regions, Yaoundé the capital city is located in the Center region where 9942 cases where recorded, with 110 deaths, respectively meaning 49.7 % of all of Cameroon cases but 26.5 % of the total national death toll. The region with the highest number of cases and the lowest lethality rate :1.1% as opposed to 7% in the North region. In general, men were more affected than women with a Male/Female gender ratio of 1.4. While the age group from 30 to 39 years old was the most affected (1684 females and 2299 males , N=14 077), mortality was higher in the 60-69 year old group (31 females and 72 males, N=325). During the week of September 9 only, 2020, 81 patients were hospitalized nationwide. The severity rate fluctuated from 0 to around 2.5% [80] while worldwide, it had been estimated that around 13,8% of infected people develop severe symptoms [81]. Depending on several factors. One hundred and twenty-four (124) hospitalized patients (severe cases) were monitored by SOS AMY and Hadassah health workers in the capital city.
EMERGENCY INTERVENTIONS MONITORED BY PHYSICIANS IN HEALTH FACILITIES
In Yaoundé, lifesaving interventions were carried out mainly in 2 public institutions, the Central Hospital (military stadium and ORCA) and the General Hospital of Yaoundé, and by private institutions including the Djoungolo health facility, Le Jourdain, Catholic health centers, Hadassah Hospitals Cameroon and SOS AMY; under heavy emergency situations created by the then high COVID-19 pandemic impact. The major confinement site was OLEMBE. In the present study, Hadassah Hospitals and SOS AMY managed by the President of the Association of Emergency Physician of Cameroon monitored hospitalized patients, out patients and their close contacts. The diagnosis of COVID-19 was based on clinical signs, the performance of PCR and antigen tests as well as on imaging examinations (CT scan and chest X-ray) in case of suspicion of the presence of the virus despite the negativity of the initial test results. During the care period, six categories of patients were observed; based on the patient treatment and/or severity of the COVID-19 case. The first category or group (grp) made of inpatients who received the Anti-COVID-19 improved traditional medicines (MTAs) Kit, Category 2 consisted of inpatients who received chloroquine treatment and category 3 was made of inpatients who received the MTA Ngul be Tara (NBT) alone. Category 4 contained out patients/diagnosed COVID-19 case monitored at home or place of confinement by SOS AMY who were treated with the Anti-COVID-19 MTA Kit; Category 5 contained outpatients/diagnosed COVID-19 cases monitored at home or confinement place by SOS AMY who received the Ngul be Tara treatment alone; Category 6 was made of COVID-19 index cases’ close contacts who were administered Ngul be Tara for prophylaxis. Each patient or health worker had received a 6 to 10-day treatment following the below protocol.
Medicines Presentation
Ngul Be Tara was available as a 500 ml liquid solution or dry powder of 30 g. Netko and Binther were available as a 60 ml and 30 ml liquid solutions, respectively. Immunoboost was provided as a 16 g dry powder in a glass bottle. Chloroquine was distributed as 100 mg tablets. Azithromycin, Amoxyclav and Ceftriazone were available as 500 mg tablets or capsules.
Protocol administered using the KIT of 4 MTAs for 6 days to 10 days
The improved traditional medicines were provided by oral route to patients as follows: Imnunoboost: 1 teaspoon in the morning just before breakfast; Netko: 1 tablespoons (15 ml) in the morning at breakfast and Dinner: Binther: 1 teaspoon (5 ml) in the morning at breakfast and lunch; three hours later, liquid Ngul be Tara : 2 tablespoons (30 ml) during lunch; 4 hours later: 2 tablespoons (30 ml) of liquid Ngul be Tara during snack; 4 hours later: 2 tablespoons (30 ml) of liquid Ngul be Tara during dinner.
Protocol of Ngul be Tara powder for 10 days
For patients receiving a curative treatment, 1 teaspoon (3 g) of Ngul be Tara powder was prescribed three times a day for adults 60 kg and over, or 50 mg/kg of body weight. For prophylaxis, the same dose was provided but only twice a day.
Protocol for Chloroquine
Patients following the standard chloroquine-based Treatment received 2 tablets of 100 mg chloroquine 3 times a day, 1 tablet of Zinc per day; 2 tablets of Vitamin C per day; 2 tablets per day of Amoxyclav; 1 tablet of Azithromycin per day for 10 days. All in-patients with severe COVID-19, displaying dyspnea and co-morbidities received antibiotic treatment such as azithromycin 1g twice a day, and/or ceftriaxone 1g every 12 hours.
GLOBAL OBSERVATIONS IN SELECTED PATIENTS AND THEIR CLOSE CONTACTS
A total of 513 participants treated with improved traditional Medicines (MTAs) and 39 with chloroquine were selected. The sample size selected was 552, with 317 males and 235 females, a male/female ratio of 1.35. The chloroquine inpatients’ group was only compared with the inpatients’ group treated with African MTAs no bigger than 5 times the former category as stated in the material and method section. Inpatients data were compared as well as out patients’ data, respectively. The average age for the initially selected sample of 552 participants was 45.65±15.3, varying between 6 and 93 years. (Tab. 1).
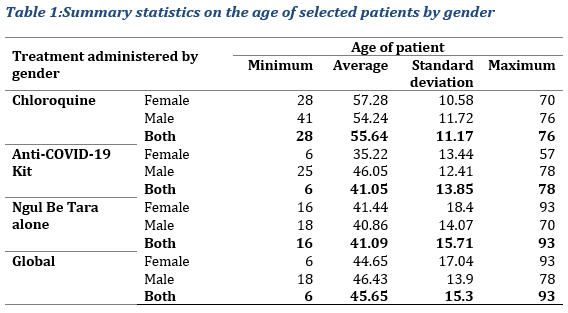
With the chloroquine treatment, the age varied between 28 and 76 years with an average of 55.64±11.17. The minimum age in this subsample was observed in female patients and the maximum age in male patients (Tab. 1). Regarding the MTAs, for the Anti-COVID-19 Kit, the observed average age of treated patients was around 41.05±13.85 years. The age in this sub-sample varies between 6 and 78 years. For treatment with Ngul Be Tara alone, the average age of the patients who were administered this MTA was 41.09±15.71; the age varied between 16 and 93 years old. The average age for patients treated with traditional medicines seemed lower because some patients were very young in age, as low as 6 years old, however, most of the patients were adults over 50 years of age.
INPATIENT DATA
The systematic selection of in-patient lead to a database of 129 patients including 5 patients under observation put on prophylactic treatment for COVID-19 at the Hadassah Hospitals (one male and four female). The treatment administered to those in-patients was Anti-Covid-19 kit for prevention (one male and four female) and the rest of in-patient received either Anti-COVID-19 kit, Ngul be Tara alone or chloroquine for treatment distributed as stated in Figure 1.
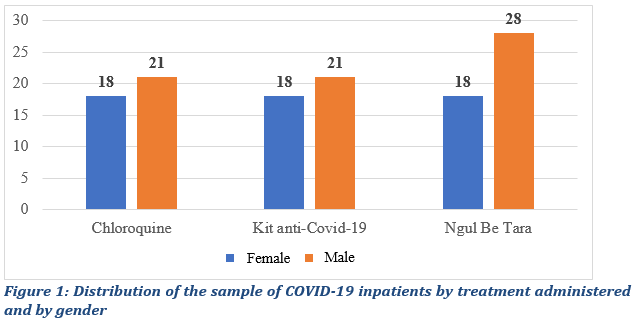
For selected inpatients, 43.5% were female patients and 56.5% men, with 39 patients treated with Chloroquine, 39 with the anti-Covid-19 Kit and 46 with Ngul be Tara alone (Fig.1). The 124 in-patients who tested positive for COVID-19 and received curative treatment were analyzed as a group. Data from these124 were compared. Beforehand, the normality and the homoscedasticity of our samples compared to the reference population were studied. This preliminary verification focused on the continuous variables, namely the age of the patient and the duration of the symptoms. To test normality, we used the Kolmogorov-Smirnov and Shapiro-Wilk tests, more efficient for small samples, as follows: H0∶ The population has a normal distribution for the characteristics (age and duration) and H1∶The samples do not come from a population that has a symmetric distribution. The test results for the two characteristics taking into account sub-samples are recorded in table 2.
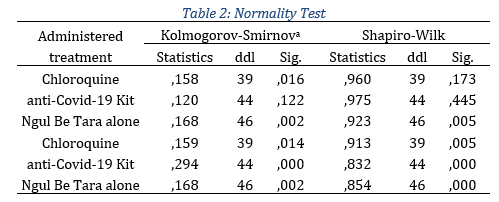
In view of the evidence, only the age distribution of patients who took the anti-Covid-19 kit suggests normality compared to the reference population. For the rest of the subsamples the null hypothesis is violated. Therefore, in the realization of comparison hypotheses tests, we had to use nonparametric tests. To test homogeneity, we used Levene's test according to the following assumptions: H0∶ Equality of the variances of the sub-samples (Homoskedasticity), H1∶ At least two variances are different (Heteroskedasticity). The test results for the two characteristics taking into account sub-samples are recorded in the table 3.
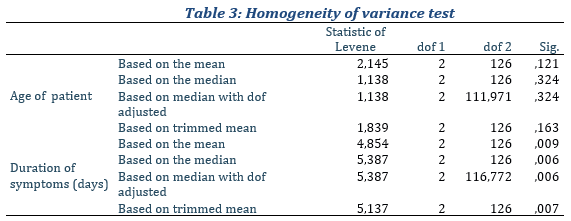
With regard to the evidence provided by our dataset (Table 3), our sub-samples have significantly equal variances for age but with regard to the duration of symptoms, the hypothesis of homoscedasticity is rejected. In other words, the resulting differences in sample variances are unlikely to have occurred on the basis of random sampling from a population of equal variances. In general, the set of prerequisites for carrying out parametric tests for comparing two independent samples are biased. Thus, to test our different results for the comparison of our samples for continuous distributions, we used nonparametric tests instead in specific cases. In the present study, for quantitative variables, imputation was done through the mean across groups.
Observed symptoms
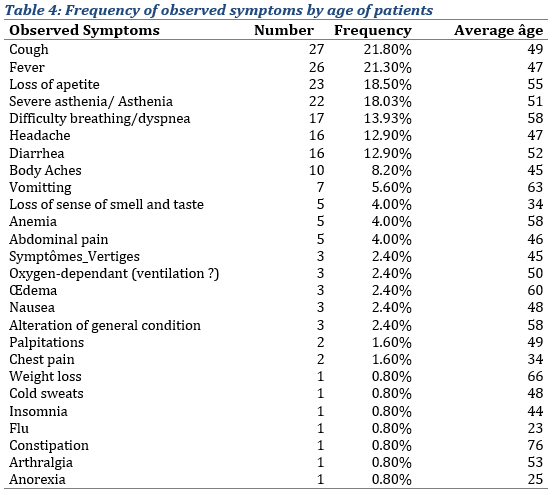
Regarding symptoms, the most common were: cough (21.80%), the average age observed in patients with this symptom was 49 years (Tab. 4); followed be fever (20.20%); loss of appetite (18.50%); asthenia (18.03%), difficulty breathing (13.93%), headache (12.90%) and diarrhea (12.90%). Less frequent symptoms such as weight loss, nausea, insomnia, constipation (1 case) were also observed. One patient could display more than one symptoms.
Presence or absence of symptoms in COVID-19 inpatients
Most of the patients, whether those placed under modern or traditional medicine, presented specific symptoms of COVID-19: 100% of the patients treated with chloroquine, 79.49% of those treated with the Anti-COVID 19 kit and 97.80% of those treated with Ngul Be Tara alone (Fig. 2). Eight patients treated with the Anti-COVID-19 kit did not display classic symptoms of COVID-19 but had other severe comorbidities (Fig. 2).
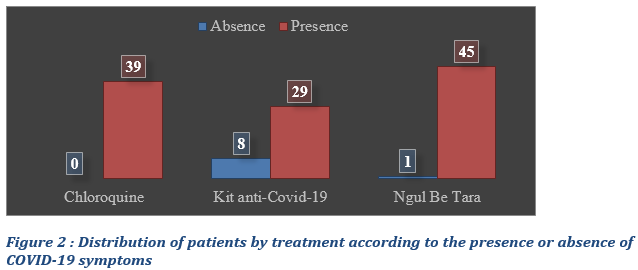
Statistical comparison between groups of patients using Chloroquine vs and anti-Covid-19 Kit and Ngul be Tara protocols respectively, with regards to the presence of symptoms
To test whether the observe difference was significant, comparison test were run. The Chi-Square and the Fisher's Exact Test (Tab.5 & 6). The null hypothesis being the equality of proportions with regards of the presence or absence of symptoms in the 3 groups.
Chloroquine vs and anti-Covid-19 Kit groups
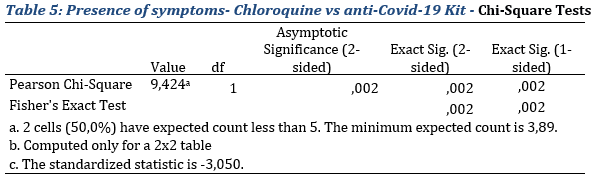
With regards to results exhibited in table 5, the null hypothesis of equality of proportions is rejected. Thus, the difference observed with regards to the presence or absence of symptoms is significant to the threshold of 5% (p-value=0,002 for the Fisher’s test which is the most suitable.
Chloroquine and Ngul Be Tara inpatient groups
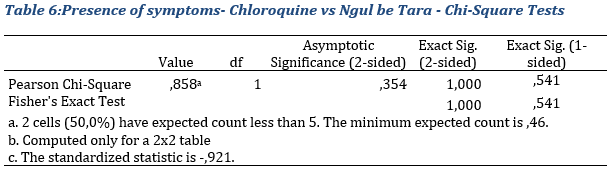
With regards to results exhibited in table 6, The fisher test comparing 2 proportions suggests a non-significant difference in the presence of symptoms between the groups treated with chloroquine and Ngul be Tara respectively. Indeed, the p-value equal to 1 that is greater that 0.05 maintains the null hypothesis.
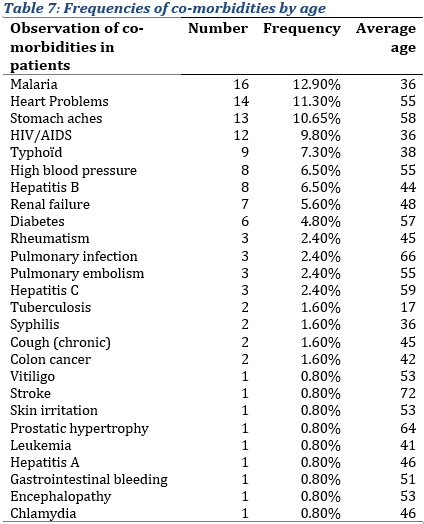
Presence of co-morbidities
The most observed co-morbidities in the sample of inpatients were malaria, cardiac and stomach problems (Tab. 7), with 16, 14 and 13 patients, respectively, having those co-morbidities (i.e. approximately 12.90%, 11.30% and 10.65% of the sample, respectively), the average age observed with these co-morbidities were respectively 36, 55 and 58 years. They were followed by HIV (9,80%), typhoid (7.30%), hepatitis B and high blood pressure (6.50% each), and kidney failure (5.60%). The rest being residual.
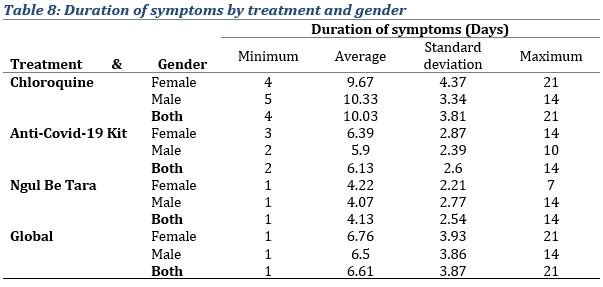
Duration of symptoms
In terms of the duration of symptoms, inpatients treated with chloroquine, symptoms seem to last longer with a duration period of 10.03 ± 2.87 days, compared to that observed in patients treated with improved traditional medicines: with the Anti-COVID-19 kit, symptoms appeared to last 6.39 ± 2.6 days and with Ngul be Tara alone, 4.13 ± 2.54 days (Tab. 8).
To analyze the difference observed with respective protocols in the duration of symptoms, non-parametric tests were used, Mann and Whitney test in this case for two samples and the Kruskal and Wallis test for more than two samples.
Duration of symptoms for Chloroquine vs Traditional medicines' protocols
Chloroquine vs anti-COVID-19 kit groups
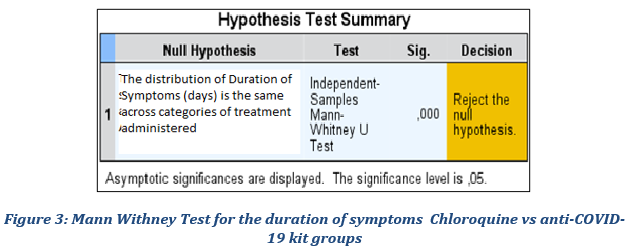
We wanted to verify whether the average duration of symptoms observed in patients treated with Chloroquine is significantly different as suggested by the descriptive statistics at the level of the reference population compared to patients treated with the anti-COVID-19 kit. For that purpose, we tested the following null hypothesis: the distribution of the duration of symptoms in the Chloroquine subgroup is identical to that of the Anti-COVID-19 Kit subgroup. The results of the test recorded in Figure 3 confirm the difference by rejecting the null hypothesis at the 5% threshold.
Chloroquine vs Ngul be Tara groups
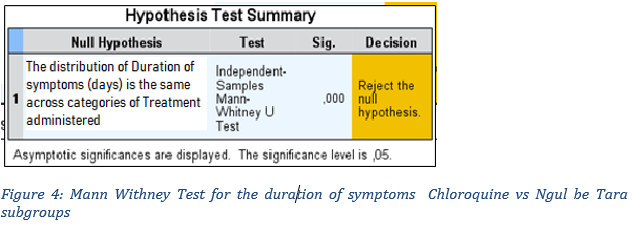
We wanted to verify whether the average duration of symptoms observed in the cohort of patients treated with Chloroquine is significantly different as suggested by the descriptive statistics at the level of the reference population compared to patients whose protocol was Ngul be Tara. We therefore tested the following null hypothesis: the distribution of the duration of symptoms in the Chloroquine subgroup is identical to that of the Ngul be Tara subgroup. The results of the test recorded in Figure 3 confirm the difference by rejecting the null hypothesis at the 5% threshold.
All selected patients for whom the treatment was curative, the initial screening test was positive regardless of the treatment provided. To analyze the performance of the different treatments in terms of SARS-COV2 eradication, patient's survival data and results of the control test following treatment were compared. The cure rate, defined as a negative test post treatment and survival, was calculated for each treatment regimen.
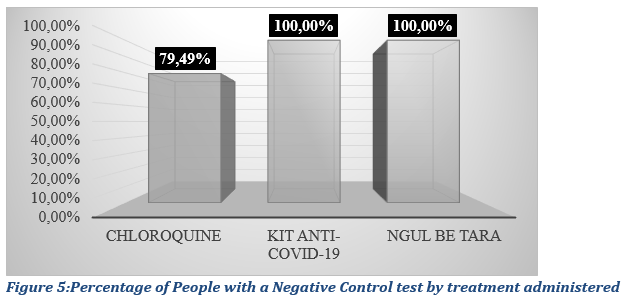
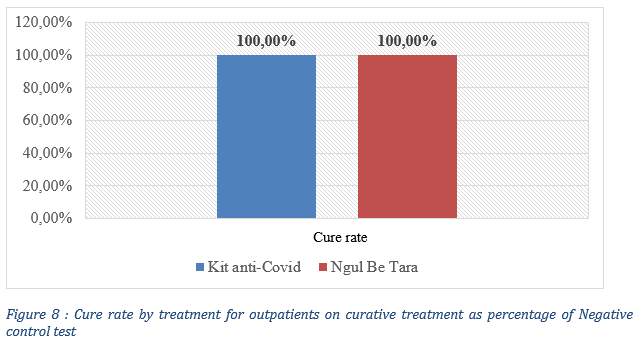
The results show that plant based traditional treatments seem to have a higher cure rate (100%) compared to that of chloroquine treatments, with a Chloroquine cure rate of 79.49% evaluated as the percentage of a negative control test post treatment ( Fig. 5).
The cure rate following different treatments seemed different. To study the significance of this difference in order to conclude on the performance of the different treatments, comparison test of the proportions (following the principle illustrated in materials and methods) were implemented and the main results summarized (Fig. 6 & 7).
By applying the principle of the proportion comparison test, while generalizing for the case on the one hand of the proportions of Chloroquine and the anti-Covid-19 Kit treatments and on the other hand of the proportions of Chloroquine and Ngul Be Tara treatments, we obtain the results summarized below in Figure 6 and 7.
Statistical evaluation of Chloroquine vs anti-Covid-19 Kit protocols’ performance
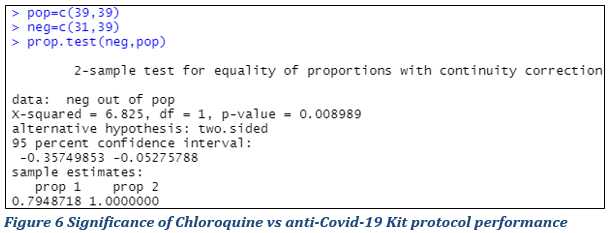
Figure 6 presents the results of a test whose null hypothesis is equality of proportions and we reject this hypothesis if the p-value of the test is less than 5% (0.05). At the 5% threshold, it is concluded that difference in the cure rates of patients on Chloroquine and Anti-covid-19 Kit treatments respectively is significant at the threshold of 5 % with the calculated p-value = 0.00008989 <0.05. The null hypothesis is rejected.
Statistical evaluation of Chloroquine vs Ngul Be Tara protocols’ performance
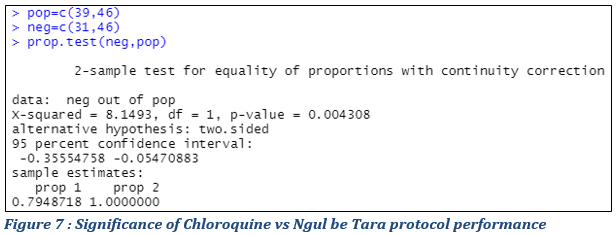
Figure 7 exhibits the results of a test whose null hypothesis is equality of proportions and we reject this hypothesis if the p-value of the test is less than 5% (0.05). At the 5% threshold, it is concluded that the cure rates of patients on Chloroquine and Ngul be Tara treatments respectively are significantly different at the threshold of 5 % with the calculated p-value of 0,004308<0.05. The null hypotlhesis is rejected.
TREATMENTS WITH IMPROVED TRADITIONAL MEDICINES (MTAS) IN SELECTED PATIENTS INCLUDING THOSE MONITORED AT HOME OR IN CONFINEMENT PLACES AND THEIR CLOSE CONTACTS
The goal was to assess the outcome of treatment with traditional medicine with a larger patient sample, in this case the 513 selected patients with 246 under curative treatment and 267 under preventive treatment.
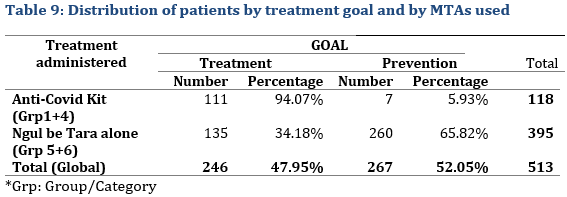
One hundred and eighteen patients used the Anti-COVID-19 kit, 111 for a curative treatment and 7 for prophylaxis,. While 395 patients used Ngul be Tara alone with 34.18% for curative treatment and 65.82% for prophylaxis (Tab. 9).
PATIENTS MONITORED FOR PREVENTION
The sample of patients monitored for prophylaxis (Tab. 10) included 116 females (43.45%) and 151 males (56.55%).
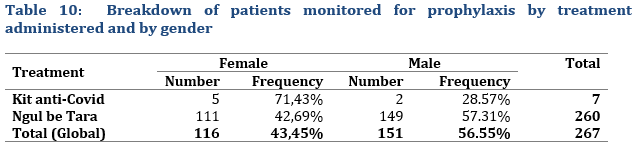
Among the overall 7 patients who were treated with the anti-Covid-19 Kit for prophylaxis, 5 patients were female, and 2 were male. For the 260 patients who were administered Ngul Be Tara alone, 42,69% (111) were female and 57.31% (149) male, a male/female ration of 1,34.
Age of patients monitored for prophylaxis
The average age of patients monitored for prophylaxis was about 39 years of age. The age ranged from 3 to 70 years (Tab. 11).
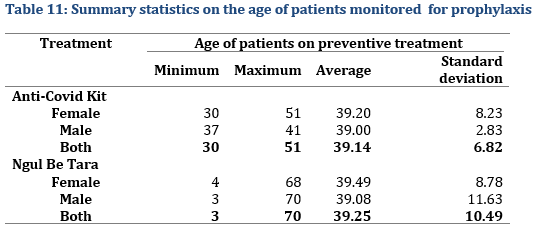
Initial Screening test for Contacts
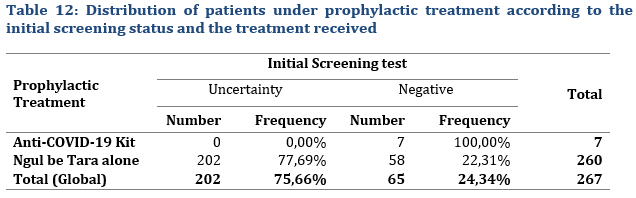
Regarding the Statistical evaluation of initial screening test, not all contacts could be tested (Tab. 12) or accepted to be tested, but rather preferred prophylactic treatment under the assumption that the contamination rate secondary infections could be high under close contact conditions. However, all close contacts of people who tested positive (index cases) all tested negative after treatment. Furthermore, none of the selected 267 treated close contacts including family members developed the disease during the over one-month observation period (Tab. 13).
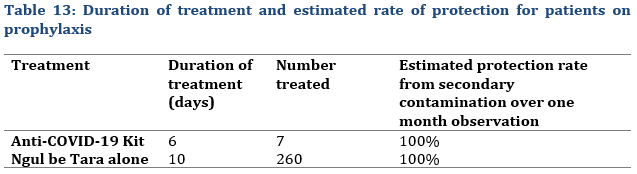
Selected patients who followed a prophylactic treatment for 6 days for the Anti-COVID-19 Kit and 10 days for Ngul be Tara alone all had a negative control test. Furthermore, no close contact within the family or other close-contact places neither tested positive nor developed COVID-19 during an over one-month observation period (Tab.13). That was defined as a 100% overall protection rate under prophylactic treatment.
OVERALL COVID-19 CASES WHO RECEIVED CURATIVE TREATMENT
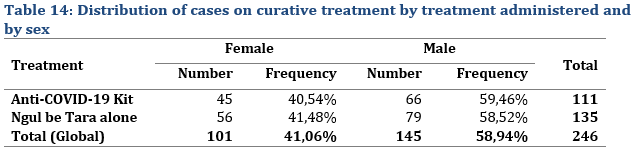
Of the 246 patients who were monitored for curative treatment, 101 (41.06%) were female and 145 (or 58.94%) were male. Male were more represented than female. The distribution was almost proportional between the treatments (Tab. 12); 40.54% of patients on Anti-COVID-19 Kit treatment were female and 59.46% male. This distribution was almost similar for the case of patients who were administered Ngul be Tara alone. As for the age of patients monitored for curative treatment, it varied between 6 and 93 years (Tab. 15) with an average of 43.41±11.05 years for patient treated with the MTA kit and 42.77±12.35 years for those treated with Ngul be Tara alone.
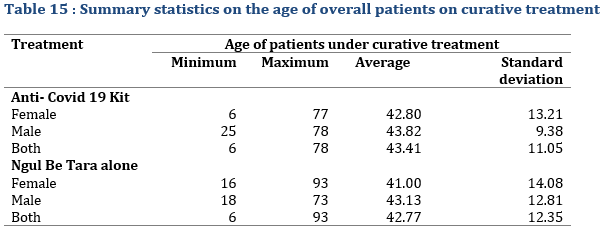
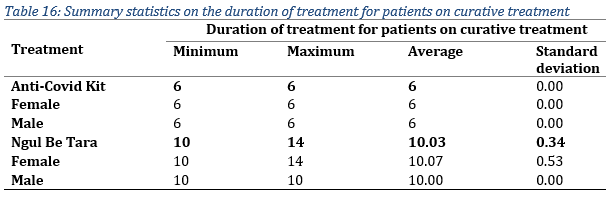
Regarding the duration of treatment administered for patients under curative treatment, it lasted up to 14 days in 1 female patient who used Ngul be Tara without respecting the dosage and frequency the first time (Tab. 16). However, the average duration for the two treatments was 6 days for the MTAs kit and 10 days for Ngul be Tara alone.
The observed cure rate was 100% for both treatments (Fig. 7), defined as a negative control test after treatment and the absence of disease symptoms. Qualitative data from health workers indicated that all patients (5000) monitored during the 3-month period all had a negative control test after treatment with improved traditional medicines under study and none of the contacts under post exposure prophylaxis neither developed COVID-19 symptoms nor tested positive for COVID-19.
REPORTED SIDE AND UNEXPECTED EFFECTS FROM IMPROVED TRADITIONAL MEDICINES
During qualitative data collection, two male participants from the selected sample reported digestive disorders while taking the medicine without meals: vomiting for one and stomach disturbance for the other. One female patient reported an appetite increase during treatment. One female patient reported post stroke sequels' improvements (increased mobility) while another reported the reversal of stroke symptoms onset (one side paralysis). One male patient reported increased sexual arousal.
SATISFACTION LEVEL
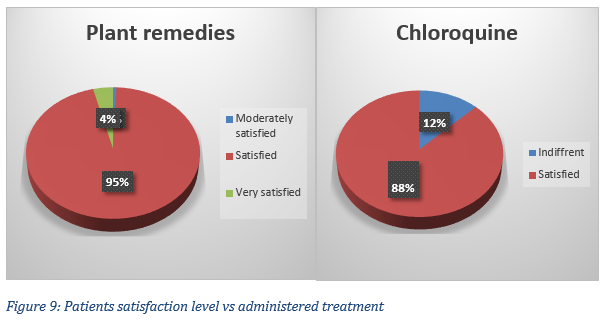
Regarding the chloroquine treatment, 28 patients out of 32 respondents were satisfied (87 %) with the treatment received, 4 were indifferent; 8 patients had deceased. In terms of MTAs, the satisfaction level of 151 respondents varied: 1 was moderately satisfied (0.7 %), 144 declared being satisfied (95 %) and 6 (4 %) were very satisfied (Fig. 9).
Discussion
Emerging viral infections generate major anxieties because they are generally caused by lethal, highly transmissible, and pathogenic viruses producing severe morbidity and mortality rates in humans. Diseases caused by coronaviruses including COVID-19, SARS and MERS significantly impact infected people as well as the economies of infected countries. As for COVID-19, there is no specific worldwide accepted drug for treatment or prophylaxis [82]. However, medicinal plants such as several African plants [83]. and Artemisia annua are considered promising treatments for COVID-19 [84,85].
Traditional medicine practice for COVID-19 seems to be used all over the world. Many governments also formally or informally advocate or authorize its use to treat COVID-19 including Cameroon; the Anti-COVID-19 MTA Kit including Ngul be Tara is an example of such officially authorized MTAs. Several studies claimed that traditional medicines could be possible candidates for the prevention and treatment of COVID-19 including local medicinal plants which can be of potential contribution to the fight against COVID-19 provided that more scientific evidence for their efficacy is made available, to establish standard formulations and clinical studies as part of efforts to develop therapies for COVID-19 [86-91].
Plant extracts and products have been shown to display antiviral activities [92] and protease inhibitors which are a class of compounds that have been extensively used in the management of viruses like HIV, MERS-CoV and SARS-CoV [93,94]. The structural and non-structural proteins essential for the life cycle of the coronavirus are proteolytically processed from the polyprotein by 3CLPRO (MPRO) and the PLPRO [95] Natural products like diarylheptanoids [96], terpenoids [97], flavonoids and coumarins [98] are potent inhibitors of the SARS-CoV proteases. In silico and in vitro analyses have found that epigallocatechin gallate, gallocatechin gallate and quercetin are potent inhibitors of the SARS-CoV-2 MPRO [99,100]. Flavonoids such as kaempferol and isoliquiritigenin synergistically inhibited the SARS-CoV-2 MPRO and PLPRO in vitro. Flavonoids from traditional Chinese medicines, like herbacetin, rhoifolin, and pectolinarin, were found to inhibit the MPRO of SARS-CoV [101]. Jo et al. found that flavonoids like herbacetin, isobavachalcone and helichrysetin have an inhibitory effect on MERS-CoV MPRO [102]. Wen et al. investigated over 200 plant extracts to find their inhibitory effect on SARS-CoV. Furthermore, SARS-CoV induced cytopathogenic effects were studied in Vero E6 cell lines and they have shown that herbal extracts proved to have a potential inhibitory effect on SARS-CoV. [103]. A recent in silico study on naturally derived compounds came up with 3 potential leads which can block the entry of the SARS-CoV-2 in the host cells by inhibiting the host target protein TMPRSS2. The same study also showed that the three compounds (glucogallin, mangiferin, and phlorizin) could also be used to restrict the virus's life cycle inside the host due to their inhibitory action on the viral MPRO [104].
WHO also recognizes traditional, complementary and alternative medicine of proven quality, safety and efficacy have many benefits [105]. Africa has a long history of traditional medicine and practitioners that play a crucial role in providing care to populations [106,107]. Such practices are required to be tested for efficacy and safety. We set out to compare the efficacy of formulated improved traditional medicines manufactured with Cameroon grown plants with that of the chloroquine regimen, a validated repurposed drug used to address COVID-19 nationwide and in many continents. Characteristics of the selected sample were similar to results published by the local Ministry of health, the average age of the infected people was 39 years of age similar to Cameroon published results with the most hit being 30-39 years old, and the ratio male/female were [80] 1.35 similar to the published national 1.4 ratio in the then 20009 recorded infected people in the country. The population studied represented ¼ of nationwide COVID-19 cases. The selected sample of people treated with the MTA kit shows a symmetric distribution. Obtained results show that people treated with plant based remedies, namely Ngul be tara, Netko Binther and Immunoboost significantly recovered almost twice as fast as those treated with the chloroquine regimen. Indeed, they all displayed a negative test after 6 to 10 days of treatment compared to those treated with the chloroquine treatments (79 % recovered). The result was statistically significant. All the monitored people who received a post exposure prophylactic treatment for 10 days had a negative control test. That is, a 100% overall protection rate under prophylaxis for all of those who would have otherwise been infected. With an estimated COVID-19 secondary attack rate of 10 to 15 % [9], one might expect around 27 to 40 contaminated close contacts from the selected sample. With a reported COVID-19 secondary attack rate of about of 58.2% during the Delta-dominant period and 80.9% during the Omicron-dominant period [11], one would expect to have 157 or 218 secondary infected contacts from our sample, respectively. However, no close contact in the family, within the hospital or other close-contact-places neither tested positive nor developed COVID-19 during an over one-month observation period. Needless to say there is also compelling evidence that traditional medicine provides beneficial effect in the treatment or prevention of SARS [108]. Thus, our results go along the line with several therapies that have been used to address COVID-19 cases with a variety of regimens as reviewed by Song Yand & al. [109]. While conventional therapies usually address one step of the disease onset and progression, plants display several active ingredients allowing them to address several steps of the disease evolution. For example, Remdesivir, lopinavir/ritonavir and umifenovir, if considered, are often initiated before the peak of viral replication for an optimal outcome. Ribavirin may be beneficial as an add-on therapy but is ineffective as monotherapy. The corticosteroids use is usually limited to address specific co-morbidities. While African plant remedies with an array of active ingredients seem to make giant steps in the treatment of COVID-19, with an array of multitarget biocompounds [110]. available naturally. The Madagascar Government made public the results of a Phase III clinical trial on the CVO+ remedy, in the form of capsules based on lyophilized extracts of Artemisia annua and other medicinal plants, which is intended for the treatment of mild and moderate forms of COVID-19 [111].
Due to their now closely monitored favorable safety profiles, natural products and phytomedicines are being explored as potential therapeutic or prophylactic agents with different mechanisms of action against COVID-19. [112]. Some natural products have the potential to impair the attachment of SARS-CoV-2 spike glycoprotein to its receptors on human cells [113]. including the Heat Shock Protein A5 substrate-binding domain β and angiotensin-converting enzyme 2 (ACE2) receptor [114,115]. Others have been proposed as inhibitors of viral replication, such as the recently reported compounds derived from Alpinia officinarum and ginger that may affect SARS-CoV-2 replication by blocking the SARS-CoV-2 papain-like protease [116], alkaloids and terpenoids derived from African plants that may inhibit the 3-chymotrypsin-like protease [117], or natural polyphenols such as quercetin that may inhibit the RNA-dependent RNA polymerase [118].
We report the efficacy of African Remedies that are rich in polyphenols, phenols, glycosides, alkaloids, terpenoids, tannins, antioxidants and minerals (data, in press) that may be able to address each step of the COVID-19 pathology development, thus strengthening and complementing most of the above stated conventional one-target specific drugs. The COVID-19 epidemic could be more efficiently stopped with a comprehensive approach using both conventional and plant based remedies.
Conclusion
African Improved Traditional medicines evaluated can efficiently treat and prevent COVID-19 infections. Results are statistically significant and indicate that African medicines can address from prevention to severe cases of SARS-COV2 infections. NBT could efficiently be used for pre or post exposure prophylaxis and for treatment for 10 days. However, post exposure treatment is recommended to avoid resistance development and waste of drugs. The AntiCOVID-19 kit may be used to treat severe cases in patients with several comorbidities efficiently It may also provide a shorter treatment time (6 days instead of 10). According to qualitative data, no MTAs treatment failure was observed by the health worker team during treatment of 5000 cases. African plant remedies show great promise for combatting COVID-19 like diseases.
Study limitations and perspectives
Despite significant results, there are limitations that need to be addressed by future work, including trials with people from a greater diversity of ethnicities, advocacy for a multination double blind randomized controlled trial using capsules. The latter can make possible to use of placebos (on asymptomatic to moderate cases) not easily adapted to traditional medicine clinical research. The aim of this study was to study the performance of improved traditional treatment against Covid-19 compared to that of the protocol of a nationally validated modern medicine protocol. The composition of groups to be compared constitutes the ultimate limit of obtained results. Due to the non-control of the choice of observation units during the interventional treatment of patients, the tests carried out could not be parametric (asymmetry of the distributions of 2 subsamples) and therefore leading to results assessment via nonparametric tests that are less robust though effective. The inference of the results on the 5000 patient sample remains valid, however inference to the overall population remains to be improved through random controlled interventional trials.
Funding
The present study was funded by the Cameroon population and the National insurance funds (CNPS). Medicines were provided by researchers from Reece International Research Consortium, a not for profit research institute promoting development.
Acknowledgment
Financial support by the National Social Insurance Fund (CNPS) and its Director Mr Mekulu Mvondo Alain Olivier funded the present studies. Improved Traditional Medicines provided for the studies by RIRCO researchers were manufactured partly with equipment donated by Oregon Health Sciences University and its Former President Mr Jim Walker.
Conflict of interest
The authors declare no conflict of interest.
References
1. Chan J.F.W., Li K.S.M., To K.K.W., Cheng V.C.C., Chen H., and Yuen K.-Y. (2012). ‘Is the discovery of the novel human betacoronavirus 2c EMC/2012 (HCoV-EMC) the beginning of another SARS-like pandemic?’ J. Infect. Dec;65(6):477–489.
2. Gallagher T.M., and Buchmeier M.J. (2001). ‘Coronavirus spike proteins in viral entry and pathogenesis’. Virology 279(2):371–374.
3. Chan, J. F.-W., Kok, K.-H., Zhu, Z., Chu, H., To, K. K.-W., Yuan, S., et al. (2020a). ‘Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan’. Emerg. Microbes and Infect. 9 (1), 221–236.
4. Krishnamoorthy, S., Swain, B., Verma, R. S., & Gunthe, S. S. (2020). ‘SARS-CoV, MERS-CoV, and 2019-nCoV viruses: an overview of origin, evolution, and genetic variations’. Virus disease, 31(4), 1–13. Advance online publication. https://doi.org/10.1007/s13337-020-00632-9.
5. Cascella M., Rajnik M., Aleem A., et al. (2021). ‘Features, Evaluation, and Treatment of Coronavirus (COVID-19)’ [Updated 2021 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; Jan-. Available at : https://www.ncbi.nlm.nih.gov/books/NBK554776/ (Accessed :10 September 2021).
6. Wang, P.; Casner, R.G.; Nair, M.S.; Wang, M.; Yu, J.; Cerutti, G.; Liu, L.; Kwong, P.D.; Huang, Y.; Shapiro, L.; et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe 2021, 29, 747–751.
7. Guo, Y.-R., Cao, Q.-D., Hong, Z.-S., Tan, Y.-Y., Chen, S.-D., Jin, H.-J., et al. (2020). ‘The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status’. Mil. Med Res. 7 (1), 1–10. doi:10.1186/s40779-020-00240-0.
8. Wu Z. and McGoogan J.M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention’. JAMA. Feb 24 doi: 10.1001/jama.2020.2648.
9. Bi Q, Wu Y, Mei S, Ye C, et al. (2020). ‘Epidemiology and transmission of COVID‐19 in Shenzhen, China: analysis of 391 cases and 1286 of their close contacts’. Lancet Infect Dis. 10.1016/s1473-3099(20)30287-5.
10. Karumanagoundar, K., Raju; M., Ponnaiah, M., Kaur, P., Viswanathan, V., Rubeshkumar, P., Sakthivel, M., et al. (2021). ‘Secondary attack rate of COVID-19 among contacts and risk factors. Tamil Nadu, March-May 2020: a retrospective cohort study’. BMJ Open. Nov 5;11(11):e051491. doi: 10.1136/bmjopen-2021-051491.
11. Muñoz L.I., Torrella A., Pérez-Quílez O., Castillo-Zuza A., Martró E., Bordoy A.E. et al. (2022). ‘SARS-CoV-2 Secondary Attack Rates in Vaccinated and Unvaccinated Household Contacts during Replacement of Delta with Omicron Variant, Spain’. Emerg Infect Dis. Oct;28(10):1999-2008. doi: 10.3201/eid2810.220494.
12. Sampath V., Rabinowitz G., Shah M., et al. (2021). ‘Vaccines and allergic reactions: The past, the current COVID-19 pandemic, and future perspectives’. Allergy. 76(6):1640-1660. doi:10.1111/all.14840.
13. WHO (2021). ‘WHO Coronavirus (COVID-19) Dashboard’. Available at- https://covid19.who.int/. (Accessed: December 12, 2021).
14. Gobeil S.M.C., Janowska K., McDowell S., Mansouri K., Parks R., Stalls V., et al. (2021). ‘Effect of Natural Mutations of SARS-CoV-2 on Spike Structure, Conformation, and Antigenicity’. Science (80 ) 373(6555):eabi6226. doi: 10.1126/SCIENCE.ABI6226.
15. Cai Y., Zhang J., Xiao T, Lavine C.L., Rawson S., Peng H., et al. (2021). Structural Basis for Enhanced Infectivity and Immune Evasion of SARS-CoV-2 Variants. Science (80) 373:642–8. doi: 10.1126/SCIENCE.ABI9745
16. McCallum M., Bassi J., De Marco A., Chen A., Walls A.C., Di Iulio J., et al. (2021). ‘SARS-CoV-2 Immune Evasion by the B.1.427/B.1.429 Variant of Concern’. Science (80) 373:648–54. doi: 10.1126/SCIENCE.ABI7994
17. Katella K. (2022). ‘Paxlovid, the Latest COVID-19 Pill’. Yale medicine. Available at https://www.yalemedicine.org/news/13-things-to-know-paxlovid-covid-19. (Accessed: 15 March, 2022).
18. Bryant A., Lawrie T.A., Dowswell T., Fordham E.J., Mitchell S., Hill S.R.,and Tham T.C. (2021). ‘Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines’. Am J Ther. Jun 21;28(4):e434-e460. doi: 10.1097/MJT.0000000000001402.
19. Coopersmith, C.M., Antonelli, M., Bauer, S.R., Deutschman, C.S., Evans, L.E., Ferrer, R. et al. (2021). ‘The Surviving Sepsis Campaign: Research Priorities for Coronavirus Disease 2019 in Critical Illness’. Crit Care Med. Apr 01;49(4):598-622.
20. Sheahan, T. P., Sims, A. C., Graham, R. L., Menachery, V. D., Gralinski, L. E., Case J. B., et al. (2017). ‘Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses’. Sci. Transl. Med. 9(396), 1–10.
21. Sheu, T. G., Deyde, V. M., Okomo-Adhiambo, M., Garten, R. J., Xu, X., Bright, R. A., et al. (2008). ‘Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008’. Antimicrob. Agents Chemother. 52(9), 3284–3292.
22. Geretti, A. M., Armenia, D. and Ceccherini,-Silberstein F. (2012). ‘Emerging patterns and implications of HIV-1 integrase inhibitor resistance’. Curr. Opin. Infect. Dis. 25(6), 677–686.
23. Locarnini, S. A. and Yuen, L. (2010). ‘Molecular genesis of drug-resistant and vaccine-escape HBV mutants’. Antiviral Ther. 15(3 Pt B), 451–461.
24. Kumar, M., Mazumder, P., Mohapatra, S., Kumar, T. A., Dhangar, K., Taki ,K., et al. (2021). ‘A chronicle of SARS-CoV-2: seasonality, environmental fate, transport, inactivation, and antiviral drug resistance’. J Hazard Mater. Mar 5;405:124043.
25. Lin, L. T., Hsu, W. C. and Lin, C. C. (2014). ‘Antiviral natural products and herbal medicines’. J. Tradit. Complement. Med. 4(1), 24–35.
26. Kumar, A., Narayan, RK, Prasoon, P, Kumari, C, Kaur, G, Kumar, S, et al. (2021). ‘COVID-19 Mechanisms in the Human Body—What We Know So Far’. Front. Immunol. 12:693938. doi: 10.3389/fimmu.2021.693938.
27. Sudre, C.H., Murray, B., Varsavsky, T., Graham, M.S., Penfold, R.S., Bowyer, R.C., et al. (2021). ‘Attributes and Predictors of Long COVID’. Nat. Med., 27:1–6. doi: 10.1038/s41591-021-01292.
28. Kumar, A., Prasoon, P., Sekhawat, P.S., Pareek, V., Faiq, M.A., Kumari, C., et al. (2020). ‘Pathogenesis Guided Therapeutic Management of COVID-19: An Immunological Perspective’. Int Rev Immunol, 40:1–18. doi: 10.1080/08830185.2020.1840566.
29. Thierry, A.R. (2020). ‘Host/genetic Factors Associated With COVID-19 Call for Precision Medicine’. Precis Clin Med, 3:228–34. doi: 10.1093/pcmedi/pbaa026.
30. Zhang, X., Tan, Y., Ling, Y., Lu, G., Liu, F, Yi, Z., et al. (2020). ‘Viral and Host Factors Related to the Clinical Outcome of COVID-19’. Nature 583:1–7. doi: 10.1038/s41586-020-2355-0.
31. Ejaz, H., Alsrhani, A., Zafar, A., Javed, H., Junaid, K., Abdalla, A.E., et al. (2020). ‘COVID-19 and Comorbidities: Deleterious Impact on Infected Patients’. J Infect Public Health, 13:1833–9. doi: 10.1016/j.jiph.2020.07.014.
32. Chung, M.K., Karnik, S., Saef, J., Bergmann, C., Barnard, J., Lederman, M..M, et al. (2020). ‘SARS-CoV-2 and ACE2: The Biology and Clinical Data Settling the ARB and ACEI Controversy’. EBioMedicine 58:102907. doi: 10.1016/j.ebiom.2020.102907.
33. Kumar, A., Prasoon, P., Kumari, C., Pareek, V., Faiq, M.A., Narayan, R.K., et al. (2020). ‘SARS-CoV-2-Specific Virulence Factors in COVID-19’. J Med Virol 93:1343–50. doi: 10.1002/jmv.26615.
34. Dhar, D., Mohanty, A. (2020). ‘Gut Microbiota and Covid-19- Possible Link and Implications’. Virus Res, 285:198018. doi: 10.1016/j.virusres.2020.198018.
35. Zuo, T., Zhang, F., Lui, G..CY., Yeoh, Y.K., Li, A.Y.L., Zhan, H., et al. (2020). ‘Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization’. Gastroenterology ,159:944–55.e8. doi: 10.1053/j.gastro.2020.05.048.
36. Tao, W., Zhang G., Wang, X., Guo, M., Zeng, W., Xu Z., et al. (2020). ‘Analysis of the Intestinal Microbiota in COVID-19 Patients and Its Correlation With the Inflammatory Factor IL-18’. Med Microecol, 5:100023. doi: 10.1016/j.medmic.2020.100023.
37. Gu, S., Chen, Y., Wu, Z., Chen, Y., Gao, H., Lv, L., et al. (2020). ‘Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza’. Clin Infect Dis 71:2669–78. doi: 10.1093/cid/ciaa709.
38. Gandhi, R.T., Lynch, J.B., Del Rio, C. (2020). ‘Mild or Moderate Covid-19’. N Engl J Med. Oct 29;383(18):1757-1766.
39. Radzikowska, U., Ding, M., Tan, G., Zhakparov, D., Peng, Y., Wawrzyniak, P., et al. (2020). ‘Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors’. Allergy, 75(11):2829-45. https://doi.org/10.1111/all.14429.
40. Mancilha, E.M.B., and Oliveira, J.S.R. (2021). ‘SARS-CoV-2 association with hemoglobin and iron metabolism’. Rev Assoc Med Bras (1992), Sep;67(9):1349-1352. doi: 10.1590/1806-9282.20210555. PMID: 34816933.
41. Barciszewska, A.-M. (2021). ‘Elucidating of oxidative distress in COVID-19 and methods of its prevention’. Chemico-Biological Interactions, Volume 344, 109501, ISSN 0009-2797,https://doi.org/10.1016/j.cbi.2021.109501.
42. De Flora, S., Balansky R., and La Maestra, S. (2020). ‘Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19’. Faseb. J., 34 (10) (Oct) pp. 13185-13193, 10.1096/fj.202001807.
43. Entrenas Castillo, M., Entrenas Costa, L.M., Vaquero Barrios, J.M., Alcalá Díaz, J.F., López Miranda, J. Bouillon, R., and J.M. Quesada Gomez (2020). ‘Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study’. J. Steroid Biochem. Mol. Biol., 203 (Oct), Article 105751, 10.1016/j.jsbmb.2020.105751
44. M.J. Keller, E.A. Kitsis, S. Arora, J.T. Chen, S. Agarwal, M.J. Ross, Y. Tomer, W. Southern. (2020). ‘Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19’. J. Hosp. Med., 15 (8) (Aug), pp. 489-493, 10.12788/jhm.3497.
45. Salles, É.L., Khodadadi, H., Jarrahi, A., Ahluwalia, M., Paffaro Jr., V.A., Costigliola, et al. (2020). ‘Cannabidiol (CBD) modulation of apelin in acute respiratory distress syndrome’. J. Cell Mol. Med., 24 (21) (Nov), pp. 12869-12872.
46. Sies, H. and Parnham, M.J. (2020). ‘Potential therapeutic use of ebselen for COVID-19 and other respiratory viral infections’. Free Radic. Biol. Med., 156 (Aug 20), pp. 107-112.
47. Hedlund, R. Diamond T.K., Uversky V.N. (2020). ‘The latitude hypothesis, vitamin D, and SARS-Co-V2’. J. Biomol. Struct. Dyn. (Jul 17), pp. 1-3.
48. Wahedi, H.M. Ahmad S., Abbasi. S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. (2020 May 12), pp. 1-10.
49. Taher, M. Tik, N. Susanti, D. (2021). ‘Drugs intervention study in COVID-19 management’. Drug Metab Pers Ther, (Apr 5), 10.1515/dmdi-2020-0173.
50. Y. Xian, J. Zhang, Z. Bian, H. Zhoun, Z. Zhang, Z. Lin, H. Xu. (2020). ‘Bioactive natural compounds against human coronaviruses: a review and perspective’. Acta Pharm. Sin. B, 10 (7) pp. 1163-1174.
51. Li, M., Zhu, H., Liu, Y., Lu, Y., Sun, M., Zhang, Y. et al. (2022). ‘Role of Traditional Chinese Medicine in Treating Severe or Critical COVID-19: A Systematic Review of Randomized Controlled Trials and Observational Studies’. Front. Pharmacol., 13:926189. doi: 10.3389/fphar.2022.926189.
52. Leung, P.-C. (2007). ‘The efficacy of Chinese medicine for SARS: a review of Chinese publications after the crisis’. Am. J. Chin. Med. 35 (04), 575–581. doi:10. 1142/s0192415x07005077.
53. Hensel, A., Bauer, R.,Heinrich,M., Spiegler, V., Kayser, O., Hempel, G., et al. (2020). ‘Challenges at the time of COVID-19: opportunities and innovations in antivirals from nature’. Planta Med., 86 (10), 659. doi:10.1055/a-1177-4396.
54. Luo, H., Tang, Q. L., Shang, Y. X., Liang, S. B., Yang, M., Robinson, N., et al. (2020). ‘Can Chinese medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs’. Chin. J. Integr. Med., 1–8.
55. Elujoba, A. A., Odeleye, O. M., and Ogunyemi, C. M. (2005). ‘Traditional medicine development for medical and dental primary health care delivery system in Africa’. Afr. J Tradit Complement Altern Med, 2 (1), 46–61.
56. Mahomoodally, M. F. (2013). ‘Traditional medicines in Africa: an appraisal of ten potent African medicinal plants’. Evid. Based Complement. Alternat Med., 2013, 617459. doi:10.1155/2013/617459.
57. Wordometer report coronavirus cases weekly trends available at https://www.worldometers.info/coronavirus. (Accessed 2020-2022).
58. WHO Coronavirus (COVID-19) Dashboard Available at https://covid19.who.int/?adgroupsurvey={adgroupsurvey}&gclid=CjwKCAiArY2fBhB9EiwAWqHK6gX9EM_U9BVexX3lKmCzI-EpTmjTEaRAizJtNP7SZe4hseI-2FrCeRoCEj0QAvD_BwE. (Accessed 2020-2022).
59. Worldometer https://www.worldometers.info/world-population/africa-population/#:~:text=Africa%20Population%20(LIVE)&text=The%20current%20population%20of%20Africa,the%20latest%20United%20Nations%20estimates. (Accessed 2020-2022).
60. The World Health Organization (2022). Over two-thirds of Africans exposed to virus which causes COVID-19: WHO study. Available at https://www.afro.who.int/news/over-two-thirds-africans-exposed-virus-which-causes-covid-19-who-study. (Accessed 2020-2022).
61. Lam K.-H., Lee K.K.-H., Gambari R., Kok S.H.-L., Kok T.-W., Chan A.S.-C., Bian Z.-X., Wong W.-Y., Wong R.S.-M., Lau F.-Y., et al. Anti-tumour and pharmacokinetics study of 2-Formyl-8-hydroxy-quinolinium chloride as Galipea longiflora alkaloid analogue. Phytomedicine. 2014;21:877–882. doi: 10.1016/j.phymed.2014.02.005.
62. Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69.
63. Lam K.-H., Gambari R., Lee K.K.-H., Chen Y.-X., Kok S.H.-L., Wong R.S.-M., Lau F.-Y., Cheng C.-H., Wong W.-Y., Bian Z.-X., et al. Preparation of 8-hydroxyquinoline derivatives as potential antibiotics against Staphylococcus aureus. Bioorg. Med. Chem. Lett. 2014;24:367–370. doi: 10.1016/j.bmcl.2013.10.072.
64. Vandekerckhove S., Tran H.G., Desmet T., D’hooghe M. Evaluation of (4-aminobutyloxy)quinolines as a novel class of antifungal agents. Bioorg. Med. Chem. Lett. 2013;23:4641–4643. doi: 10.1016/j.bmcl.2013.06.014.
65. Ratheesh M., Sindhu G., Helen A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013;62:367–376. doi: 10.1007/s00011-013-0588-1.
66. Kouznetsov V., Vargas Mendez L., Milena Leal S., Mora Cruz U., Andres Coronado C., Melendez Gomez C., Romero Bohorquez A., Escobar Rivero P. Target-Oriented Synthesis of Antiparasitic 2-Hetaryl Substituted Quinolines Based on Imino Diels-Alder Reactions. Lett. Drug Des. Discov. 2007;4:293–296. doi: 10.2174/157018007784620031.
67. Rolain J.-M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
68. Zheng X., Wang L., Wang B., Miao K., Xiang K., Feng S., Gao L., Shen H.C., Yun H. Discovery of Piperazinylquinoline Derivatives as Novel Respiratory Syncytial Virus Fusion Inhibitors. ACS Med. Chem. Lett. 2016;7:558–562. doi: 10.1021/acsmedchemlett.5b00234.
69. Talamas F.X., Abbot S.C., Anand S., Brameld K.A., Carter D.S., Chen J., Davis D., de Vicente J., Fung A.D., Gong L., et al. (2014). 'Discovery of N-[4-[6-tert-Butyl-5-methoxy-8-(6-methoxy-2-oxo-1-H-pyridin-3-yl)-3-quinolyl]phenyl]methanesulfonamide (RG7109), a Potent Inhibitor of the Hepatitis C Virus NS5B Polymerase'. J. Med. Chem.,57:1914–1931. doi: 10.1021/jm401329s.
70. Goodell J.R., Puig-Basagoiti F., Forshey B.M., Shi P., Ferguson D.M. Identification of Compounds with Anti-West Nile Virus Activity. J. Med. Chem. 2006;49:2127–2137. doi: 10.1021/jm051229y.
71. Ezgimen M., Lai H., Mueller N.H., Lee K., Cuny G., Ostrov D.A., Padmanabhan R. Characterization of the 8-hydroxyquinoline scaffold for inhibitors of West Nile virus serine protease. Antivir. Res. 2012;94:18–24. doi: 10.1016/j.antiviral.2012.02.003.
72. Talamas, F.X., Abbot, S.C., Anand ,S., Brameld, K.A., Carter, D.S., Chen, J., Davis, D., de Vicente, J., Fung, A.D., Gong, L., et al. (2014). ‘Discovery of N-[4-[6-tert-Butyl-5-methoxy-8-(6-methoxy-2-oxo-1-H-pyridin-3-yl)-3-quinolyl]phenyl]methanesulfonamide (RG7109), a Potent Inhibitor of the Hepatitis C Virus NS5B Polymerase’. J. Med. Chem., 57:1914–1931. doi: 10.1021/jm401329s.
73. Huang, S.-H., Lien, J.-C., Chen, C.-J., Liu, Y.-C., Wang, C.-Y., Ping, C.-F., Lin, Y.-F., Huang, A.-C., and Lin, C.-W. (2016). ‘Antiviral Activity of a Novel Compound CW-33 against Japanese Encephalitis Virus through Inhibiting Intracellular Calcium Overload’. Int. J. Mol. Sci.,17:1386. doi: 10.3390/ijms17091386.
74. Barbosa-Lima, G., Moraes, A.M., da Araújo ,A.S., da Silva, E.T., de Freitas, C.S., Vieira, Y.R., Marttorelli ,A., Neto, J.C., Bozza, P.T., de Souza, M.V.N., et al. (2017). ‘2,8-Bis(trifluoromethyl)quinoline analogs show improved anti-Zika virus activity, compared to mefloquine’. Eur. J. Med. Chem.,127:334–340.
75. Lai, H., Sridhar Prasad, G., and Padmanabhan, R. (2013). ‘Characterization of 8-hydroxyquinoline derivatives containing aminobenzothiazole as inhibitors of dengue virus type 2 protease in vitro’. Antivir. Res.;97:74–80. doi: 10.1016/j.antiviral.2012.10.009.
76. Gogtay, N.J. (2010). ‘Principles of sample size calculation). Indian J Ophthalmol. Nov-Dec;58(6):517-8. doi: 10.4103/0301-4738.71692. PMID: 20952836; PMCID: PMC2993982.
77. Grosofsky, A. (2009). ‘Statistics Decision Aids - Decision Tree for inferential statistics’. Available at. https://columbusstate.libguides.com/ld.php?content_id=51641307/ (Accessed: August 30 2020).
78. Médecins Sans Frontières MSF(2020). Available at https://www.msf.org/msf-supports-covid-19-response-cameroon (Accessed: 16 April 2020).
79. Worldometer global COVID-19 statistics (2020). Available at https://www.worldometers.info/coronavirus/country/cameroon/. (Accessed: August 30 2020).
80. Ministry of public health Cameroun (2020). « Centre de coordination des urgences de santé publique ». ‘COVID-19 Situation report’. Available at https://www.ccousp.cm/wp-content/uploads/2021/12/CMR_COVID19_SITREP47.pdf. (Accessed: December 12, 2021).
81. United States Centers for Disease Control and Prevention (CDC) ‘Symptoms of Novel Coronavirus (2019-nCoV)’. Available at https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. (Accessed: December 12, 2021).
82. Tegen, D., Dessie, K., and Damtie, D. (2021). ‘Candidate anti-COVID-19 medicinal plants from Ethiopia: a review of plants traditionally used to treat viral diseases’. Evidence-Based Compl Alternative Med. Jun 4; 6622410. doi: 10.1155/2021/6622410.
83. Umeta Chali, B., Melaku, T., Berhanu, N., Mengistu, B., Milkessa, G., Mamo, G., Alemu, S., and Mulugeta, T. (2021). ‘Traditional Medicine Practice in the Context of COVID-19 Pandemic: Community Claim in Jimma Zone, Oromia, Ethiopia’. Infect Drug Resist.,14:3773-3783 https://doi.org/10.2147/IDR.S331434.
84. Orege, J.I., Adeyemi, S.B., Tiamiyu, B.B., Akinyemi, T.O., Ibrahim, Y.A., and Orege, O.B. (2021). ‘Artemisia and Artemisia-based products for COVID-19 management: current state and future perspective’. Adv Traditional Med.,1–12. doi:10.1007/s13596-021-00576-5.
85. Haq, F.U., Roman, M., Ahmad, K., et al. (2020). ‘Artemisia annua: trials are needed for COVID-19’. Phytother Res., 34:2423–2424. doi:10.1002/ptr.6733.
86. Vellingiri, B., Jayaramayya, K., Iyer, M., et al. (2020). ‘COVID-19: a promising cure for the global panic’. Sci Total Environ.,725:138277. doi:10.1016/j.scitotenv.2020.138277.
87. Rastogi, S., Pandey, D.N., and Singh, R.H. (2022). ‘COVID-19 pandemic: a pragmatic plan for ayurveda intervention’. J Ayurveda Integr Med., Jan-Mar;13(1):100312. doi: 10.1016/j.jaim.2020.04.002.
88. Yang, F., Zhang, Y., Tariq, A., et al. (2020). ‘Food as medicine: a possible preventive measure against coronavirus disease (COVID‐19)’. Phytother Res.,34(12):3124–3136. doi:10.1002/ptr.6770.
89. El Alami, A., Fattah, A., and Chait, A. (2020). ‘Medicinal plants used for the prevention purposes during the covid-19 pandemic in Morocco’. J Analytical Sci Appl Biotechnol., 2(1):1–2.
90. Khadka, D., Dhamala, M.K., Li F, et al. (2021). ‘The use of medicinal plants to prevent COVID-19 in Nepal’. J Ethnobiol Ethnomed., 17(1):1–7. doi:10.1186/s13002-021-00449-w.
91. Fongnzossie Fedoung, E., Biwole, A.B., Nyangono Biyegue, C.F. Ngansop Tounkam, M., Akono Ntonga P., Nguiamba V.P., et al. (2021). ‘A review of Cameroonian medicinal plants with potentials for the management of the COVID-19 pandemic’. Adv Tradit Med (ADTM) 2021 Mar 26:1–26. doi: 10.1007/s13596-021-00567.
92. Chakravarti, R., Singh, R., Ghosh, A., Deya, D., Sharmaa, P., Velayuthama, R. et al. (2021). ‘A review on potential of natural products in the management of COVID-19’. RSC Adv., 11, 16711-16735. DOI: 10.1039/D1RA00644D.
93. De Wit, E., Van Doremalen, N., Falzarano, D., and. Munster, V. J. (2016). ‘SARS and MERS: recent insights into emerging coronaviruses’. Nat. Rev. Microbiol., 14(8), 523.
94. Ghosh, A. K., Osswald, H. L. and Prato, G. (2016). ‘Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS’. J. Med. Chem., 59(11), 5172–5208.
95. Dai, W., Zhang, B., Jiang, X. M., Su, H., Li, J., Zhao, YXie, X., Jin, Z, Peng, J., Liu, F. and Li, C. (2020). ‘Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease’. Science, 368(6497), 1331–1335.
96. Park, Y., Jeong, H. J., Kim, J. H., Kim, Y. M., Park, S. J., Kim, D., Park, K. H., Lee, W. S. and Ryu, Y. B. (2012). ‘Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus’. Biol. Pharm. Bull., 35(11), 2036–2042.
97. Park, J. Y., Kim, J. H., Kim, Y. M., Jeong, H. J,. Kim, D. W., Park, K. H., Kwon, H. J., Park, S. J. Lee, W. S. and Ryu, Y. B. ‘Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases’. Bioorg. Med. Chem., 2012, 20(19), 5928–5935.
98. Park, J. Y., Ko, J. A., Kim, D. W., Kim, Y. M., Kwon, H. J. Jeon, H. J., Kim, C. Y., Park, K. H. Lee, W. S. and. Ryu, Y. B . (2016). ‘Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV’. J. Enzyme Inhib. Med. Chem., 31(1), 23–30.
99. Nguyen, T. T., Woo, H. J., Kang, H., K. Kim, Y. M., Kim, D. W. Ahn, S. A Xia Y. and Kim, and D. (2012). Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris, Biotechnol. Lett,. 34(5), 831–838.
100. Ghosh, R., Chakraborty, A,. Biswas, A. and Chowdhuri S. (2020). ‘Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors – an in silico docking and molecular dynamics simulation study’. J. Biomol. Struct. Dyn., 1–3.
101. Kim, S., Jo, S., Shin, D. H. and Kim, M. S. (2020). ‘Inhibition of SARS-CoV 3CL protease by flavonoids’. J. Enzyme Inhib. Med. Chem., 35(1), 145–151.
102. Jo, S.; Kim, H., Kim, S., Shin, D. H. and Kim, M. S. (2019). ‘Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors’. Chem. Biol. Drug Des., 94(6), 2023–2030.
103. Wen, C. C., Shyur, L. F, Jan, J. T., Liang, P. H., Kuo, C. J., Arulselvan, P., Wu, J. B., Kuo, S. C. and Yang, N. S. (2011). ‘Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication’. J. Tradit. Complement. Med., 1(1), 41–50.
104. Singh, R., Gautam, A., Chandel, S., Ghosh, A., Dey, D., Roy, S., Ravichandiran, V., and Ghosh, D. (2020). ‘Protease Inhibitory Effect of Natural Polyphenolic Compounds on SARS-CoV-2: An In Silico Study’. Molecules, 25(20), 4604 105. World Health Organization (2013). ‘WHO traditional medicine strategy: 2014–2023’. Available at https://apps.who.int/iris/handle/10665/92455. (Accessed : 13 December 2021).
106. Ezekwesili-Ofili J.O. and Okaka A.N.C. (2019). Herbal Medicines in African Traditional Medicine, Herbal Medicine, Philip F. Builders, IntechOpen. Available at: https://www.intechopen.com/books/herbal-medicine/herbal-medicines-in-african-traditional-medicine. (Assessed 10 December, 2019).
107. Ozioma, E.O., and Chinwe, O.N. (2019). ‘Herbal medicines in African traditional medicine’. Herbal Med., 30(10):191–214. doi: 10.5772/intechopen.80348.
108. Yang, Y., Islam, M. S., Wang, J., Li, Y., & Chen, X. (2020). ‘Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective’. International journal of biological sciences, 16(10), 1708–1717. https://doi.org/10.7150/ijbs.45538.
109. Song, Y., Zhang, M., Yin, L., Wang, K., Zhou, Y., Zhou, M., and Lu, Y. (2020). ‘COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2)’. Int J Antimicrob Agents, Aug;56(2):106080. doi: 10.1016/j.ijantimicag.2020.106080. Epub 2020 Jul 4. PMID: 32634603; PMCID: PMC7334905.
110. Xiana,Y., Zhanga, J., Bianc, Z., Zhoud, H., Zhanga, Z., Lina, Z., and Xud, H. (2020). ‘Bioactive natural compounds against humancoronaviruses: a review and perspective’. Acta Pharmaceutica Sinica B;10(7):1163e1174.
111. Boakye-Agyemang, C. (2021). ‘WHO statement on the clinical trial of CVO+ remedy’. Antananarivo / Brazzaville - The World Health Organization. Availaible at https://www.afro.who.int/news/who-statement-clinical-trial-cvo-remedy (Accessed: 10 July 2020).
112. Huang, J., Tao, G., Liu, J., Cai, J., Huang, Z., and Chen, J. X. (2020). ‘Current prevention of COVID-19: natural products and herbal medicine’. Front Pharmacol. 11, 588508. doi:10.3389/fphar.2020.588508
113. Fatih, M.U., Saran, S., Hitesh, W., and Vuong T (2021). ‘Repurposing Anti-Malaria Phytomedicine Artemisinin as a COVID-19 Drug’. Frontiers in Pharmacology VOL 12 https://www.frontiersin.org/article/10.3389/fphar.2021.649532 . DOI=10.3389/fphar.2021.649532.
114. Elfiky, A. A. (2020). ‘Natural products may interfere with SARS-CoV-2 attachment to the host cell’. J. Biomol. Struct. Dyn. doi:10.1080/07391102.2020.1761881.
115. Kumar, V., Dhanjal, J. K., Bhargava, P., Kaul, A., Wang, J., Zhang, H., et al. (2020). ‘Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells’. J. Biomol. Struct. Dyn. doi:10.1080/07391102.2020.1775704.
116. Goswami, D., Kumar, M., Ghosh, S. K., and Das, A. (2020). ‘Natural product compounds in Alpinia officinarum and ginger are potent SARS-CoV-2 papain-like protease inhibitors’. ChemRxiv. Cambridge: Cambridge Open Engage; This content is a preprint and has not been peer-reviewed.
117. Gyebi, G. A., Ogunro, O. B., Adegunloye, A. P., Ogunyemi, O. M., and Afolabi, S. O. (2020). ‘Potential inhibitors of coronavirus 3-chymotrypsin-like protease (3CL pro): an in silico screening of alkaloids and terpenoids from African medicinal plants’. J. Biomol. Struct. Dyn. doi:10.1080/07391102.2020.1764868.
118. El-Aziz Abd, N. M., Mohamed, G. S., Awad, O. M. E., and El-Sohaimy, S. A. (2020). ‘Inhibition of COVID-19 RNA-dependent RNA polymerase by natural bioactive compounds: molecular docking analysis’. Preprint. doi:10.21203/RS.3.RS-25850/V.
Author information
1 SOS AMY Medical Center, Yaoundé Cameroon
2 National Association of Accident and Emergency Specialists, AMUCAM,Yaoundé Cameroon
3 Hadassah Hospitals Cameroon, Simbog, Yaoundé
4 Investigations Department, Sub-regional Institute of Statistics and Applied Economics, ISSEA Yaoundé Cameroon
5 Pharmacie le Cygne, Yaoundé Cameroon
6 School of Biomedical Sciences, Faculty of Sciences, University of Dschang, Cameroon
7 Department of Microbiology, Faculty of Sciences, University of Yaoundé I, Cameroon
8 Department of Political Sciences, Faculty of Political and Legal Sciences, University of Yaoundé II, Soa Cameroon
9 Department of Public Health, Faculty of Allied Health Sciences, King David University of Medical Sciences, Uburu. Ebonyi State, Nigeria