Preliminary Analysis and antioxidant activity of the African medicine Ngul be Tara traditionally used for the management of cardiovascular and respiratory diseases including Covid-19
Cite: Adebisi, F., Edoun, F.E.L., Nwaze, E.O. and Marlyse M. Peyou Ndi, M.M. (2022). 'Preliminary Analysis and antioxidant activity of the African medicine Ngul be Tara traditionally used for the management of cardiovascular and respiratory diseases including Covid-19.'The Int. J. Holist. Sci. Innov. Dev., 11(3), pp. 89–114.
Preliminary Analysis and antioxidant activity of the African medicine Ngul be Tara traditionally used for the management of cardiovascular and respiratory diseases including Covid-19
Fagbohun Adebisi1, Ferdinand E.L. Edoun2, Eric O. Nwaze3, and Marlyse M. Peyou Ndi4,5
Corresponding author: Ferdinand Edoun, edouferdinand@gmail.com - Accepted: October 2022 - Published: November 2022ABSTRACT
Ngul be Tara (NBT), is used in Cameroon traditional medicine to prevent or treat respiratory, cardiovascular and inflammatory illnesses including COVID-19. However, there was no published information on its overall phytochemical content and properties able to support its ethnomedical use. To characterize its bioactive constituents and evaluate some of its properties, preliminary physicochemical, microbiology and quality control analyses were performed., as well as antioxidant capacity assessment using the 2,2–diphenyl–1–picrylhydrazyl (DPPH) inhibition, the Phosphomolybdenum and the Ferric Reducing/Antioxidant Power (FRAP) methods. The product passed the odor, color, purity and solubility quality control tests. Terpenoids, Tannins, Coumarins, glycosides, alkaloids, flavonoids, phenols, and free quinones were present on phytochemical screening. Not only did NBT contain minerals such as iron, zinc, potassium, calcium, and magnesium but was also found to be of satisfactory microbiological quality while harboring a dose dependent antioxidant capacity. Preliminary results revealed an extract total polyphenol content, expressed as microgram of catechin equivalent per gram of dry matter (µg CaE/g DM) of 871,62 ± 34,41 and 138,29 ± 14,33 for the methanol and the water extracts respectively. The flavonoid content varied from 29,65 ± 0,78 microgram quercetin equivalent per gram of dry matter (µg QE/g DM) for the water extract to 46,31 ± 2,34 µg QE/g DM for the methanol extract. The alkaloid content was estimated at 271,32 ± 10,38 µg quinine equivalent per gram of dry matter (µg QiE/g DM) for the methanol extract and 16,93 ± 4,79 µg QiE/g DM for the water extract. These studies, therefore, have provided some preliminary biochemical basis sustaining the ethno-medical use of NBT in the treatment and prevention of cardiovascular, respiratory and other diseases.
Key words: Ngul be Tara, viral diseases, antioxidant, COVID-19, traditional medicines, cardiovascular diseases
1. INTRODUCTION
The Ngul be Tara (NBT) remedy is classified as a category II improved traditional medicines. The remedy, contemporary classified as alternative or complementary medicine is prepared from 4 major components namely Alstonia boonei, Enantia chlorantha, Guibourtia tessùannii, Euphorba hirta and minor ingredients. A wide array of chemical compounds has been isolated from Alstonia boonei. These include alkaloids, tannins, iridoids, and triterpenoids [1]. Alkaloids isolated from the plant include, but are not limited to echitamine, echitamidine, voacangine and akuammidine, Nα-formylechitamidine, and Nα-formyl-12-methoxyechitamidine [2,3]. The plant parts are rich in other bioactive compounds such as boonein, loganin, lupeol, ursolic acid and β-amyrin among which the alkaloids and triterpenoids form a major portion of the medicines [4]. Therapeutically, the bark of Alstonia boonei has been found to possess antirheumatic, anti-inflammatory, analgesic/pain-killing, antimalarial, antipyretic, antidiabetic (mild hypoglycaemic), antihelminthic, antimicrobial and antibiotic properties. [5-8]. The various ethnomedicinal, chemical, pharmacological, and toxicological properties of Alstonia boonei were reviewed and the profile revealed it is useful in the treatment and management of several illnesses [9].
Bark essential oils of Enantia chlorantha are mainly rich in sesquiterpenes with major constituents being: 1,5-Epoxysalvial-4(14)-ene (12.3%), the carbonyl derivative salvial-4(14)-ene-1-one (2.4%) and three other oxygenated sesquiterpenes, caryophyllene and humulene epoxides (respectively 13.4% and 8.1%), along with spathulenol (7.0%) [10]. Its main constituents are α-Copaene, β-Elemene, α-Gurjunene, β-Caryophyllene, α-Humulene, γ-Muurolene, Germacrene D, α-Muurolene, Cubebol, γ-Cadinene, δ-Cadinene, Calacorene, 1,5-Epoxysalvial-4(14)-ene, Spathulenol, Caryophyllene oxide, Salvial-4(14)-ene-1-one, Humulene epoxide II, α-Cadinol and Cadalene. Studies on Enantia chlorantha have reported its use in conditions such as rickettsia fever, cough, wounds, typhoid fever and infective hepatitis or jaundice [11]. It was also revealed that the plant possesses antipyretic [12] as well as antimicrobial, antimalarial, [13-15] antiviral [16] and antidiabetic activities [17]. In Cameroon, the stem bark extract of E. chlorantha is widely used to treat jaundice and urinary tract infections [18]. It had also been reported to treat leprosy spots, as hemostatic agent, and as uterine stimulant [19]. The bark of Enantia chlorantha has been used by traditional medical practitioners in Nigeria for the treatment of skin, gastric and duodenal ulcers [20].
The Photochemical studies performed on G. tessmannii revealed the presence of bioactive compounds such as tannins, phenolics, flavonoids, triterpenoids and alkaloids [21,22]. Additionally, various active biocompounds, such as antioxidants, abesotin and selenium, have been reported. In Cameroon, the stem bark of G. tessmannii is widely used against hypertension [23,24], gonorrhea, some cancers and as sexual enhancer. The modulation of the intracellular calcium ions by G. tessmannii and selenium may indicate a beneficial effect on steroidogenesis, including testosterone production [25]. The plant is one of the most abundantly used medicinal plant in Central Africa for many purposes such as the treatment of cardiovascular diseases [21,22,26].
E. hirta has been studied intensely and several active constituents have been isolated. The abundant presence of phytochemicals in whole plant of E. hirta like flavonoids, alkaloids, saponins, resins, sterols, steroids, acidic compounds, tannins, glycosides, anthraquinone, phenols and terpenoids was reported by Kader et al. [27]. Alcoholic solvents have been commonly used to extract polyphenols from natural sources [28]. Afzelin, quercitrin, and myricitrin have been isolated from the methanolic extract of E. hirta [29]. More chemical investigation of E. hirta has led to the isolation of additional compounds such as rutin, quercetin, euphorbin-A, euphorbin-B, euphorbin-C, euphorbin-D, 2,4,6-tri-O-galloyl-β-d-glucose, 1,3,4,6-tetra-O-galloyl-β-d-glucose, kaempferol, gallic acid, protocatechuic acid [30], β-amyrin, 24-methylenecycloartenol, β-sitosterol, heptacosane, nnonacosane [31], shikmic acid, tinyatoxin, choline, camphol, and quercitol derivatives containing rhamnose and chtolphenolic acid [32]. Fingerprint obtained by gas chromatography followed by mass spectrometry of Euphorbia hirta produced twenty-two “common peaks” ; of these components, the 8 most intense, corresponding to 53.317% (w/w) of the E. hirta extract, were 2-butanone, 3,3-dimethyl-1(methylsulfonyl)-O-[(methylamino)carbonyl]oxime, 2-furancarboxaldehyde, 5-(hydroxymethyl), 1,2,3-benzenetriol, 1,3,4,5-tetrahydroxycyclohexane carboxylic acid, hexadecanoic acid, 9,12,15- Octadecatrienoic acid, stigmast- 5-en-3-ol and 9,19-cyclolanost- 24-en-3-ol.
The sedative, anxiolytic, analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta have been reported in the literature [33]. The plant is used in the treatment of gastrointestinal disorders (diarrhea, dysentery, intestinal parasitosis...), explained partly by the presence of quercitrin, a flavanoid glycoside with antidiarrheal activity [34,35]; in the treatment of bronchial and respiratory diseases (asthma, bronchitis, hay fever, etc.) since it is reported to have a relaxation effect on respiration [36], and in the treatment of conjunctivitis. Hypotensive and tonic properties are also reported in E. hirta [37]. The alcoholic extract of whole plant showed hypoglycemic activity in rats [32]. The stem sap is traditionally used in the treatment of eyelid styes and a leaf poultice is used on swelling and boils [38]. Extracts of E. hirta have been found to show anticancer activity [39]. The aqueous extract of the herb strongly reduced the release of prostaglandins I2, E2, and D2 [40]. The use of the latex to facilitate the removal of thorns from the skin is common, hence the name Kulu Bifes in Cameroon. E. hirta leaf extracts had been reported to increase urine output and electrolytes in rats [41]. Several studies revealed that E. hirta possesses galactogenic, antianaphylactic, antimicrobial (antiviral [42]), antioxidant, antifeedant, antiplatelet aggregation, aflatoxin inhibition, antifertility, anthelmintic, antiplasmodial, antiamoebic, antimalarial and larvicidal properties [37-42]. In Ayurveda medicine, the decoction of dry herbs is used for skin diseases while decoction of fresh herbs is used as gargle for the treatment of thrush; the root decoction is believed to be beneficial for nursing mothers deficient in milk and for snake bites [31]. The root exudate exhibits nematicidal activity against juveniles of Meloidogyne incognita. E. hirta has also been used to treat Dengue fever [43]. The polyphenolic extract is responsible for E. hirta antiamoebic [44]. and antispasmodic activities [45]. It also has a sedative effect on the genitor-urinary tract [46].
Several of the above mentioned molecules have different action mechanisms, such as enzyme inhibition, chelation of trace elements involved in the production of free radicals, reactive species uptake and activation or increase in protection through other antioxidant defenses [47]. Phenolic compounds have been shown to have protective effects on humans when the plants are consumed [48]. While antioxidant capacity of phenols in plant extracts is effective at low concentrations, in humans, it is associated with the prevention of cardiovascular diseases and cancer [49-51]. Studies for the determination of the antioxidant activity of different plant remedies could contribute to establishing their value as a source of new antioxidant medicines [52,53]. Despite the ancestral and millennium long reported use of NBT ingredients, the mixture needed to be not only analyzed, but also tested for its potential value as a source of heeling phytochemicals. Our goal was to initiate the characterization of the phytochemical content and the antioxidant activity of both water and methanol extracts of the African traditional remedy NBT.
2. MATERIALS AND METHODS
2.1. Chemical reagents
The major chemical reagents were obtained from Merck: Follin-Ciocalteu [(HO)3C6H2CO2, H2O], Sodium Carbonate (Na2CO3;), Gallic Acid (C7H6O5), Aluminum Chloride (AlCl3), Potassium Acetate (CH3COOK), Quercetin (C15H10O7, 2H2O), Ethanol 99%, Chloridric acid (HCl, 12 N), Iron chloride (FeCl3), Ascorbic acid (C6H8O6), Sulfuric acid (H2SO4 ; 98%), Sodium dihydrogen phosphate (NaH2PO4), Dibasic hydrogen phosphate (Na2HPO4), Ammonium molybdate (H24Mo7N6O24), Potassium ferricyanide [K3Fe(CN)6], and Trichloroacetate (CCl3CO2Na).
2.2. Extract Preparation
Twenty grams of the product was weighed out, and 200 ml of methanol (for the methanol extract) or water (for the methanol extract) was added to each of these 20-g samples. They were left to macerate for 24 h at room temperature. Then, the samples were filtered with grade 1 Whatman paper. The methanol or water was respectively removed by evaporation. The resulting extracts were stored for later analysis.
2.3. Physicochemical and Phytochemical analysis
Phytochemical and microbiological analyses were performed by Three institutions. First by the National Laboratory of Quality Control and Expertise of Cameroon (LANACOME) and Sheda Science and Technology Complex Abuja, Nigeria and then by the National Institute of Medicinal Plants, Cameroon. Several recommended biochemistry, biophysics, and microbiology techniques were used, including WHO Quality Control Methods for Medicinal Plant Materials, WHO Geneva, 1998 [54], US Pharmacopeia National Formulary 2017: USP 40 NF 35 (United States Pharmacopeia Convention) [55], and the Codex Alimentarius international standards, codes of practice, guidelines, and recommendations [56].
2.3.1. Physicochemical analysis
The various physicochemical parameters were evaluated which includes organoleptic characteristics (color, texture and odor); the physical appearance, the presence in the product of foreign matter and purity (sand, glass particles, dirt and mold) or signs of decay; and the presence of insects.
Total Ash Value content. About 2 g of the product was incinerated in a tarred silica crucible at a temperature set at 550°C until free from carbon. It was cooled and weighed. The percentage of ash with reference to the air-dried plant material was calculated.
Water Soluble Ash content. Total ash was divided into two portions, the first portion was boiled for 5 min with 25 ml of water; insoluble matter was collected in a Gooch crucible or on an ashless` filter paper, washed with hot water, and ignited for 15 min. at 550 °C. The difference in the weight of the insoluble matter and the weight of ash represented the water-soluble ash. The percentage of water-soluble ash was calculated with reference to the air-dried plant material.
Acid insoluble Ash content. To the crucible containing total ash, 25 ml of dilute HCl was added. The insoluble matter was collected on an ashless filter paper (Whatmann number 41) and washed with hot water until the filtrate was neutral. The filter paper containing insoluble matter was transferred to a pre-weighed crucible and dried on a hot plate and ignited to constant weight. The residue was allowed to cool in a suitable desiccator for 30 min. and weighed without delay. The content of the insoluble ash was calculated with reference to the air-dried plant material.
2.3.2. Phytochemical Analysis
NBT Extracts were subjected to preliminary phytochemical screening as follows:
Quality Test for alkaloid. Few ml of 1 % v/v hydrochloric acid and NBT ethanolic extract were boiled for few minutes, warmed and filtered. Followed with addition of six drops of Mayor’s reagents (Mercuric chloride + Potassium iodide) in water. The presence of alkaloids is specified by the formation of yellow colored precipitates.
Estimation of alkaloids content. Quantification of Pentacyclic Oxindole Alkaloid (POA) extracts was performed by the method described by Singh et al. (2004), with some modifications. Briefly, 100 mg of each extract was subjected to extraction in 10 mL of ethanol solution (80%). The mixture was well homogenized and centrifuged at 5000 rpm for 10 min. After centrifugation, 1 mL of the supernatant of each extract was taken and introduced into a test tube, followed by the respective addition of 1 mL of acidified FeCl3 (0.025 M) solution (0.5 M HCl) and 1 mL of an ethanolic solution of 1,10-phenanthroline (0.05 M). The whole mixture was again incubated at 100 °C in a water bath for 30 min. The absorbance of the reddish complex formed was read at the wavelength of 510 nm against the blank. Quinine at the concentration of 25 µg/mL was used as the primary standard and the alkaloid content was expressed as µg quinine equivalent per gram of dry matter (µg QiE/g DM).
Froth test for saponins. NBT ethanolic extract was diluted with distilled water and this was shaken for 15 minutes in graduated cylinder. The presence of saponin indicated by the formation of 1 cm layer of foam.
Test for Glycosides. To 2 ml of NBT ethanolic extract, 1 ml glacial acetic acid and 1-2 drops of FeCl3 were added followed by 1 ml of concentrated H2SO4. A brown ring at the interface indicates the presence of a deoxysugar characteristic of cardenolides. A violet ring may appear below the brown ring, while in the acetic acid layer a greenish ring may form just above the brown ring and gradually spread throughout this layer
Quality Test for phytosterols. (Salkowski’s test), chloroform and concentrated sulfuric acid representing Salkowski's reagent were used. The solution of chloroform and NBT ethanolic extract were prepared and subsequently treated with concentrated sulfuric acid [57], shaken gently and allowed to stand. A positive test exhibits two distinct layers in a test tube; the upper layer (chloroform) gets blueish red to violet color, while the layer of sulfuric acid becomes yellow to green, with greenish glow being visible.
Quality Test for flavonoids. NBT ethanolic extract was treated with 2-3 drops of sodium hydroxide solution. Acute yellow color formation indicates presence of the flavonoids. The addition of few drops of concentrated sulphuric acid that changes the mixture to colorless was also sought.
Estimation of total flavonoid content. The colorimetric method described by Aiyegoro and Okoh in 2010 using Aluminium chloride was used to estimate total flavonoids. Briefly, 0.5 mL of each extract (4 mg/mL) was added to 1.5 mL of methanol, subsequently 0.1 mL of aluminium chloride (AlCl3, 10%), 0.1 mL of potassium acetate (CH3COOK, 1M), and 2.8 mL of distilled water were added. The mixture was well homogenized and incubated for 30 min at room temperature (25 ± 2 °C) and the absorbance was read at 415 nm wavelength against the reagent blank. Quercetin (0-1000 µg/mL) was used as a reference and the total flavonoid content was expressed as microgram quercetin equivalent per gram dry matter (µg QE/g DM).
Quality Test for Tannins. 2 ml of NBT ethanolic extract was mixed to 2 ml of 5% FeCl3 and the formation of yellow brown precipitate was sought.
Quality Test for Terpenoids. To 2 ml of NBT ethanolic extract, 5 ml CHCl3, 2 ml acetic anhydride, and concentrated H2SO4 were added. Reddish brown coloration of interface was monitored to detect the presence of terpenes.
Quality Test for Anthraquinones. 0.5 g of the NBT powder was boiled with 10 ml of H2SO4 and filtered while hot. The filtrate was shaken with 5 ml of CHCl3. The CHCl3 layer was pipetted into another test tube and 1 ml of dilute ammonia was added. The resulting solution was observed for color changes.
Quality Test for Phenolics. To 2 ml of NBT ethanolic extract, 1 ml of 1% ferric chloride solution was added. Blue or green colour indicates the presence of phenols.
Estimation of total polyphenols content. Total polyphenols were estimated by the modified method described by Singleton and Rossi in 1965 using the Folin-ciocalteu reagent [58]. Briefly, 0.1 ml of NBT extract (4 mg/ml) was mixed with 0.75 mL of Folin-ciocalteu reagent (10-fold dilution). The entire mixture was incubated at room temperature (25 ± 2 °C). Five min later, 0.75 mL of a sodium carbonate solution (Na2CO3, 6%) was added. The mixture was homogenized and incubated for 90 min at room temperature (25 ± 2 °C; in the dark) and then the absorbance was measured at 760 nm against a reagent blank. Gallic acid (0-1000 µg/ml) was used as a reference and the total polyphenol content was expressed as microgram of catechin equivalent per gram of dry matter (µg CaE/g DM).
Atomic Absorption Spectrophotometry (AAS) analysis for metal content. At the Sheda Science and Technology Complex Abuja Nigeria, AAS analysis of herbal powder was achieved using AOAC 2000, 985.30, 1 gram of herbal powder was weighed using analytical balance (Mettler Toledo) into a conical flask and 20 ml of 60% nitric acid was added and placed on a hot plate set at 80οC inside the fume cupboard and digested until the fume coming from the flask was clear. The digested sample was filtered into 100 ml standard volumetric flask and made up to the mark using distilled/de-ionized water. The digested solution was analyzed for metal of interest using iCE 3000 thermo Scientific Atomic Absorption Spectrophotomer with specific hallow cathode lamp for each of the metal. Concentrations of different metal in the digest sample were later determined by corresponding standard calibration curves obtained by using standard grade solutions of the elements.
Determination of the mineral content. At the National Institute of Medicinal Plants Yaoundé Cameroon, the principle was based on calcination at 450 °C and extraction of the ash with nitric acid (1N) followed by atomic absorption spectrophotometric determination of the minerals. The influence of the integrated absorbance in wavelength on the sensitivity and the precision for the determination of Ca, Fe and Zn was carried out in 3 passages according to the sequence air/acetylene flame. Measurements were performed on the main atomic lines for Fe (248.327 nm) and Zn (213.857 nm), on the secondary lines for Ca (239.856 nm), using a wavelength selected absorbance equivalent to 3 pixels for the minerals (Ca, Fe, Mg, K, Na and Zn). Recovery tests for spiked samples were performed by adding appropriate aliquots of 5000, 1000, or 100 mg/l of single standard solution to the sample digests to obtain extracts containing 10 mg/l Ca, 0.5 mg/l Fe, and 0.2 mg/l Zn. The limits of detection (LOD) and limits of quantification (LOQ) for all analytes were calculated according to IUPAC recommendations.
2.4. Antioxidant Analysis
2.4.1. 2, 2–diphenyl–1–picrylhydrazyl (DPPH) inhibition
The DPPH inhibition was used to evaluate the antioxidant activity of the product. The free-radical scavenging activity was evaluated by accessing its discoloration of 2,2-diphenyl-1-picrylhydrozyl radical (DPPH) in methanol, by using a slightly modified method of Brand-Williams et.al (1995). The following concentrations of the water and methanol extracts were tested (0.1, 0.3 0.5, 0.7, 0.9, 1.0 mg/ml). The decrease in absorbance was monitored at 517nm. Vitamin C was used as the antioxidant standard at concentrations (0.1, 0.3, 0.5, 0.7, 0.9, 1.0 mg/cm3). For a concentration of 0.1 cm3, 0.2 cm3of the extract was placed in a test-tube and 1.8 cm3 of ethanol was added followed by an addition of 0.5 cm3of 1mM of DPPH in methanol. The same was repeated for the other concentrations using the dilution formula C1V1 = C2V2. Where C1 the concentration of the stock (plant extract) = 1 mg/cm3 and V2 the volume of the dilute solution = 2 cm3 in all cases. A blank solution was prepared containing 2 cm3 of methanol and 0.5 cm3 of DPPH. Optical absorbance of the sample and blank solutions were using the UV- Visible spectrophotometer at a wavelength of 517 nm. The scavenging activity on the DPPH radical was expressed as inhibition percentage using the following equation: % Inhibition = [(Acontrol − Asample)/Acontrol] × 100 where Acontrol is the absorbance of the control reaction (containing all reagents except the test extract or standard), and Asample is the absorbance of the test extract or standard.
2.4.2. Determination of Antioxidant Activity Using the Ferric Reducing/Antioxidant Power (FRAP).
The FRAP assay was conducted following the method described by Benzie et al. [59]. Aliquots of 0.2 mL of each extract (at four different concentrations: 0.1, 0.5, 1, and 2 mg/mL; three replicates per sample and concentration) had 3.8 mL of FRAP reagent added. This reagent was previously prepared by mixing 10 parts of 300 mM sodium acetate buffer solution at pH 3.6, 1 part of 10 mM TPZT, and 1 part of 20 mM FeCl3 hexahydrate. The resulting mix was incubated for 30 min at 37 °C. The absorbance increase was measured at 593 nm in a UV/VIS spectrophotometer. The blank was prepared by substituting the same amount of diluted extract with methanol. The results were expressed in milligram equivalents of FeSO4 per milligram of dry weight. The calibration line was established using the following concentrations of FeSO4: 0.0025, 0.005, 0.01, and 0.02 mg/mL.
2.4.3. Total Antioxidant Capacity by the Phosphomolybdenum Method.
The total antioxidant capacity (TAC) of the crude extract was evaluated by the phosphomolybdenum method according to the procedure described by Prieto et al. [60]. An aliquot 300 μL (2 mg/ml) of the crude methanolic or water extract was added in 3 mL of reagent solution containing (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The mixture solution was subjected to incubation in a water bath at 95 °C for 90 min. The experiment was run in triplicates.The absorbance was measured at 695 nm with UV/VIS spectrophotometer. The total antioxidant capacity was expressed as Ascorbic acid equivalents (μg AAE/mg).
2.5. Microbiological analysis
The general microbiological quality of the product was assessed by the Enumeration of Mesophilic Total Aerobic flora FAMT for three days. From dilutions 10-1, 10-2 and 10-3, 1 ml of each dilution were put aseptically in an empty and numbered Petri dish (number of sample and dilution), 15 ml of Plate Count Agar melted and cooled to 45±1°C were added. After standardizing the inoculums on the agar, plates were incubated at 30°C for 72 h. Counting Petri dishes was based on the standards set by Standard Operating Procedures. Enumeration of Total Yeasts and Molds was also carried out, DLMT Test recommended by membrane filtration and plate counting.
2.6. Mycotoxins’ analysis
Mycotoxins’ analysis was performed according to described methods [61].
2.7. Statistical analysis.
Most Experiments were performed in triplicates, and data were expressed as mean ± Standard deviation.
3. RESULTS
3.1. Physico-chemical properties
All data were assessed using quality control limits set by International standards values (CODEX). The products characteristics are summarized in table 1. The value “passed test or not” were assigned according to the products characteristics. The tested product NBT passed the odor, color, purity and solubility tests. The crude fiber level, total ash, water soluble and acid soluble ash values fall within the CODEX limits.
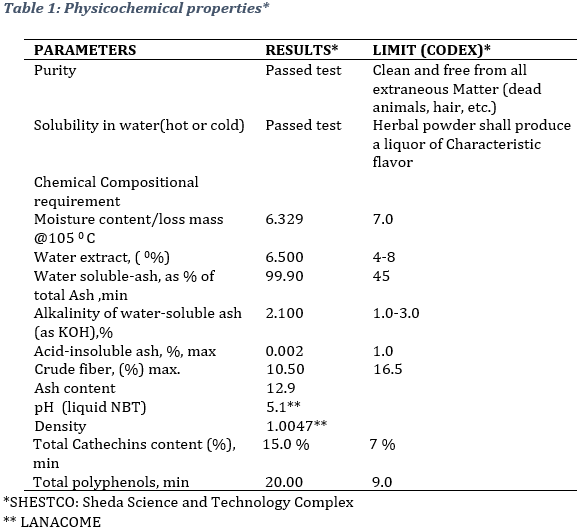
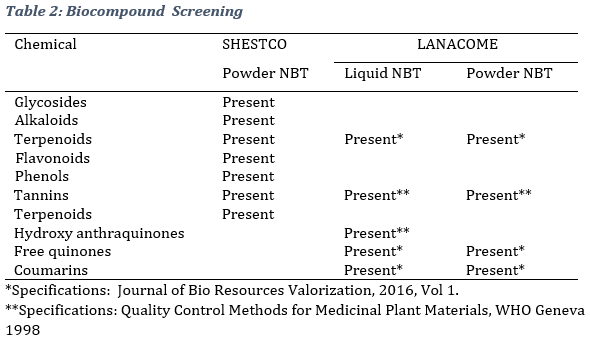
3.2. Phytochemical analysis As can be observed, phytochemical screening revealed the presence of several bioactive compounts, namely Phenols, Terpenoids, Tannins, Coumarins, Glycosides, Alkaloids, Flavonoids, and free quinones (Table 2).
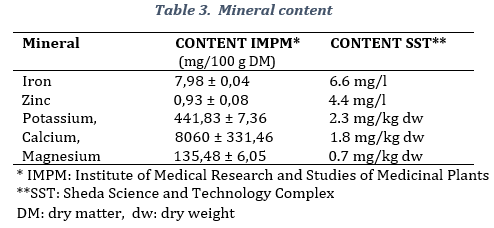
The product contained minerals such as calcium, 8060 ± 331,46 mg/100 g, potassium, 441,83 ± 7,36 mg/100 g DM, magnesium, 135,48 ± 6,05 mg/100 g DM, iron 7,98 ± 0,04 mg/100 g DM, and zinc mg/100 g DM (Table 3). All laboratories stated the presence of the same minerals, however, values were different according to the different methodologies used.
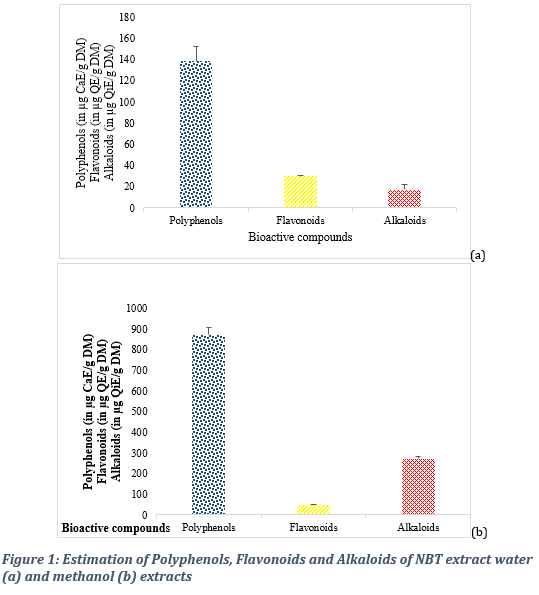
3.3. Comparative analysis of bioactive components in the water and methanol extracts Quantitative analyses carried out revealed biocompound content yields that varied from one solvent to another and from one method to another. At the medicinal Institute, the total polyphenol content expressed as microgram of catechin equivalent per gram of dry matter (µg CaE/g DM) was 871,62 ± 34,41 for the methanol extract and 138,29 ± 14,33 for the water extract (Figure 1 a & b). The polyphenol content was evaluated at 20 mg QE /mg dw) at the Sheda Science and Technology Complex (Table 1). The flavonoid content varied from 29,65 ± 0,78 microgram quercetin equivalent per gram of dry matter (µg QE/g DM) for the water extract to 46,31 ± 2,34 µg QE/g DM for the methanol extract. The alkaloid content was estimated at 271,32 ± 10,38 µg quinine equivalent per gram of dry matter (µg QiE/g DM) for the methanol extract and 16,93 ± 4,79 µg QiE/g DM for the water extract.
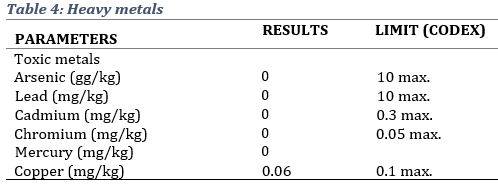
The presence of metals and toxins that have the potential to cause human or environmental toxicity was assessed. Results showed that the product conformed to CODEX limits in the terms of arsenic, lead, Cadmium, Chromium, Mercury and Copper, and aflatoxins (Table 4).
3.4. Microbiological analysis
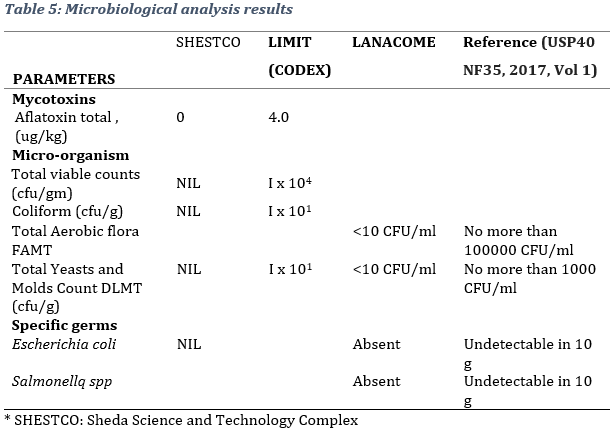
According to obtained results, the product obeyed microbiological quality norms with regard to the specifications of USP 40 NF35, 2017 and other international stands. The results for Total viable counts (cfu/gm), Coliform (cfu/gm), Yeast and Mould Count (cfu/g), Escherichia coli (cfu/gm) were NIL and classified according to international standards (Table 5).
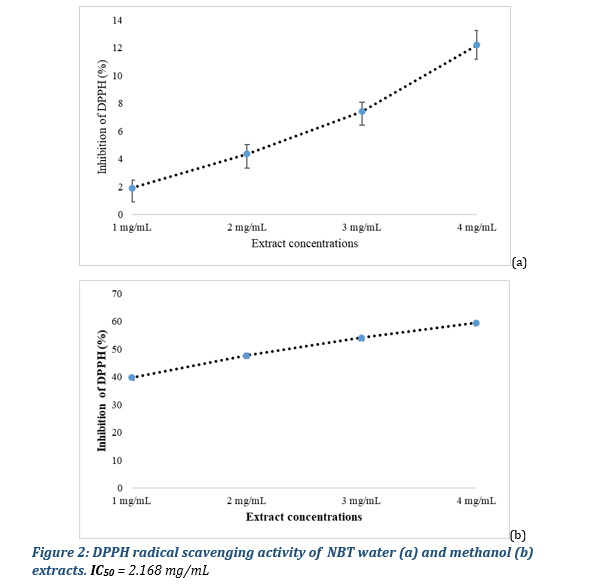
3.5 Antioxidant potential 3.5.1. Scavenging of DPPH Radical
Extracts were evaluated for their power to reduce DPPH. At all the concentrations tested, the water and methanol extracts inhibited the radical DPPH˙ in a dose-dependent manner. The results revealed a higher reducing power of the methanol extract compared to that of the water extracts (Figure 2). The methanol extract at the concentration of 1, 2, 3, and 4 mg/ml gave respective DPPH radical scavenging activity of 39,8 ± 0,3, 47,7 ± 0,8, 54,1 ± 0,1 and 59,5 ± 0,6 per cent.
The antioxidant capacity of the methanol crude extract was evaluated at 92 % compared to ascorbic acid.
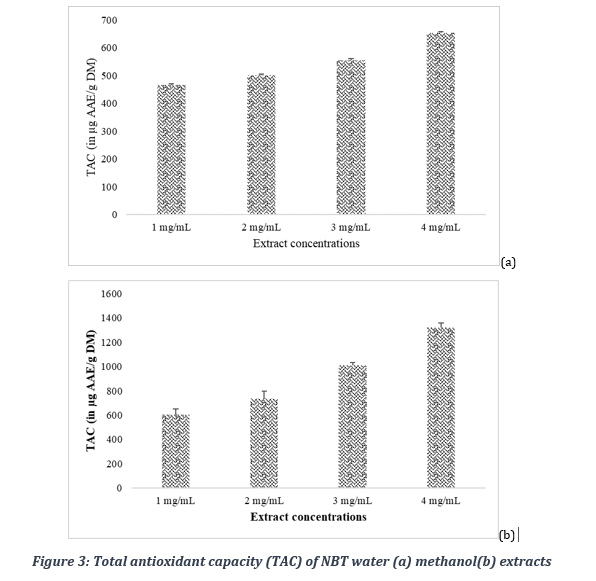
3.5.2. Total antioxidant capacity
Total antioxidant capacity of crude water extract was evaluated by the phosphomolybdenum method and was expressed as ascorbic acid equivalent per mg of plant extract. The results reveal a higher total antioxidant capacity of the methanol extract compared to that of the water extracts as shown in Figure 3. Indeed, the methanol extract of at the concentration of 1, 2, 3, and 4 mg/ml gave respective total antioxidant capacity of 603,2 ± 46,1, 736,1 ± 61,5, 1007,2 ± 26,7 and 1319,6 ± 39,8 µg AAE/g DM.
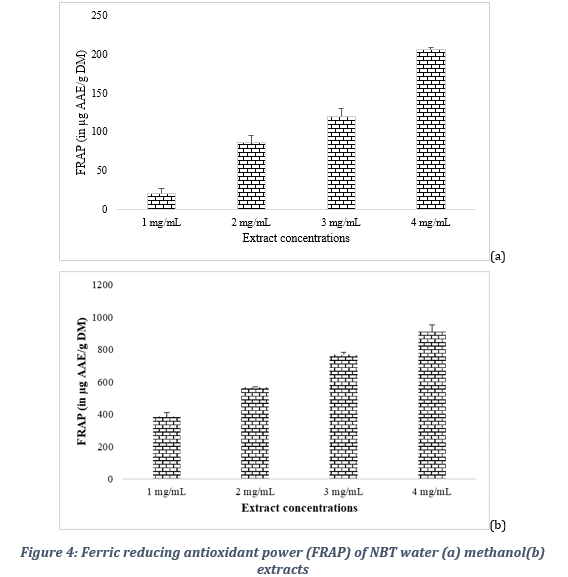
3.5.3. Ferric reducing antioxidant potential
The comparative evaluation of the reducing power of the extracts also showed a better activity of the methanol extract compared to that of the water extracts as shown in Figure 4. Indeed, the methanol extract at the concentration of 1, 2, 3 and 4 mg/ml gave respective reducing powers of 386,9 ± 21,6, 565,6 ± 3,8, 768,7 ± 14,7 and 908,7 ± 44,9 µg AAE/g DM.
4. DISCUSSION
In these studies, physicochemical, phytochemical and antioxidant evaluations were carried out to get more insights into an African medicine NBT used for the management of cardiovascular and respiratory diseases including COVID-19. NBT was found to contain non-negligible levels of Phenols, Flavonoids, terpenoids, alkaloids, tannins, coumarins, glycosides. As a result of the presence of these secondary metabolites, NBT displays high healing potential.
Polyphenols are thought to display their antioxidant capacity depending on the hydroxylation status of their aromatic rings, including (i) scavenging of free radicals, (ii) chelation and stabilization of divalent cations, and (iii) modulation of endogenous antioxidant enzymes [62].
Flavonoids are able to reduce the incidence of upper respiratory tract infections (URTIs) because they have a range of physiologic effects in humans, including antiviral, anti-inflammatory, cytotoxic, antimicrobial, and antioxidant [63]. It was noted in the present studies that NBT has a good antioxidant capacity. Moreover, the anti-inflammatory potential of flavonoids may contribute to the alleviation of flu-like symptoms. This is proven in previous studies were flavonoids supplementation decreased upper respiratory tract infections incidence by 33% compared with control, with no apparent adverse effects [64]. Studies report flavonoids have both an antiproliferative and antireplicative effect on 2 common viral sources of URTIs and reduce inflammation by decreasing NF-κB [65-68]. Secondary metabolites such as terpenes have a wide range of medicinal uses with their proven antiplasmodial, antiviral, anticancer, antidiabetic, anti-inflammatory, antioxidant, anticancer, antiseptic, antiplasmodial, astringent, digestive, diuretic, and many other properties [69]. Earlier studies have reported tannins to be responsible for high antiviral activity [42].
In previous studies, several biological activities associated with alkaloids have been reported, including analgesic [70], antibacterial [71], antifungal [72], anti-inflammatory [73], anticancer [74], and antiviral [75] activities. Among the alkaloids that have antiviral activity, berberine has shown activity against the chikungunya virus, human cytomegalovirus (HCMV), and hepatitis C virus (HCV) [76-78] tomatidine against dengue virus (DV) [79], michellamine B against human immunodeficiency virus (HIV) [80], oxymatrine against influenza A virus [81].
In the current coronavirus infection, alkaloids rich medicines provide a rich source of important natural compounds with great potential as novel anti-coronavirus agents [82]. These coumpounds have been shown to be able to inhibit viral replication in vitro, modulate host factors instead of directly targeting viral factors, inhibit 3C-like protease activity, inhibit cythopathic effect in vitro, block viral translocation through the endolysosomal system, inhibit virus-induced cell death, suppress the expression of viral S and N proteins, exert virocidal activity, block viral RNA genome synthesis, inhibit papain-like protease 2, target viral RNA and inhibit viral replication, decrease viral RNA levels, and specifically block viral replication [83,84].
Tannins are anticarcinogenic and antimutagenic properties that are believed to be related to their antioxidative activity, which is important in protecting cellular oxidative damage, including lipid peroxidation. The antimicrobial activities of tannins are well documented; the growth of many fungi, yeasts, bacteria, and viruses was inhibited by tannins [85].
Previous studies have also shown that natural coumarins are plant-derived natural products known for their pharmacological properties such as anti-inflammatory, anticoagulant, antibacterial, antifungal, antiviral, anticancer, antihypertensive, antitubercular, anticonvulsant, antiadipogenic, antihyperglycemic, antioxidant, and neuroprotective [86].
It has been demonstrated that phenolic acids, readily absorbed through intestinal tract walls, are beneficial to human health due to their potential antioxidants and avert the damage of cells resulting from free-radical oxidation reactions; on regular eating, phenolic acids also promote the anti-inflammation capacity of human beings [87].
Glycosides are vital phytochemicals in diets and are of great general interest due to their diverse bioactivity. For example, cardiac glycosides have demonstrated great efficacy in numerous heart ailments such as congestive heart failure and arrhythmia; alcoholic glycosides have displayed anti-inflammatory, antipyretic and analgesic effects; thioglycosides have allelopathic and antiseptic effects; phenolic glycosides have shown urinary tract antiseptic effect; anthocyanin have antirheumatic, antiseptic and anti-inflammatory properties, Flavonoid glycosides strengthen blood capillaries by decreasing its fragility and have antioxidant properties, among other proven properties [88,89].
These above cited biologically active constituents of NBT are indicators to its activity. In addition to the phytochemicals present, NBT is also a source of macro elements such as iron, zinc, potassium, calcium, and magnesium as detected in its ingredients during previous studies [90]. Indeed, the present study verifies the findings of Akinmoladun et al. in 2007 [90] where ingredients of NBT such as A. boonei was found to contain important minerals like calcium, phosphorus, iron, sodium, potassium and magnesium, alkaloids, tannins, saponins, flavonoids and cardiac glycosides, detected together with the important vitamin, ascorbic acid. The revealed that DPPH (2,2- diphenyl-1-picrylhydrazyl) radical scavenging activity, total phenolic content and reducing power of that plant extract were 41.58 ± 1.43 %, 2.09 ± 0.04 mg/g gallic acid equivalent and 0.32 ±± 0.01 respectively. Previous findings that support the proven potential antioxidant capacity of NBT. The medicinal effects of plants have often been attributed to the antioxidant activity of their photochemical constituent [90]. A synergistic relationship amongst phytochemicals has been cited to be responsible for the overall beneficial effect derivable from plants [91]. It has been suggested that the mineral elements present in plants may play roles in the medicinal value of the plants [90].
While further analyses remain to be done to improve the present findings, we have provided some preliminary biochemical insights into the ethnomedical use of NBT in the treatment and prevention of cardiovascular and respiratory viral diseases, including COVID-19. Indeed, during traditional therapy sessions, NBT have been found to significantly reduce COVID19 symptoms duration in index cases and block secondary infections in post exposure treated close contacts(data in press).
ACKNOWLEDGEMENTS
Financial support by the National Social Insurance Fund (CNPS) and its Director Mr Mekulu Mvondo Alain Olivier is highly appreciated. We thank Oregon Health Sciences University for equipment donation by the Former President Mr Jim Walker. We are grateful to LANACOME, the National Laboratory for Quality Control of Medicines and Expertise and its Director Dr Rose Ngono Mballa for its support running partially subsidized quality control test. The participation of Reece International Research Consortium (RIRCO) researchers in the development of endogenous solutions and subsequent research is highly appreciated.
CONFLICT OF INTEREST
RIRCO is a not for profit community based organization promoting research and the development of improved science based natural indigenous medicines to help fight epidemics and global public health threats. RIRCO researchers provide assistance in both the development of improved traditional medicines and related research in resource limited settings.
REFERENCES
1. Babatunde, O., Godwin, R. E. and Wahab, N. O. (2016). ‘Gas chromatography–mass spectrometry (GC-MS) analysis of leaf, stem-back and root extracts of Alstonia boonei’. Academic Journal conference proceedings. Article Number: 9E02872. Available at https://academicjournals.org/proceeding/CSN/article-full-text-pdf/9E02872. (Accessed 12 December 2020).
2. Kucera, M. Marquis, V. O.and Kucerova, H. (1972). ‘Nigerian medicinal plants. I. TLC thin-layer chromatographic separation and quantitative evaluation of Alstonia boonei alkaloids’. Planta Medica, vol. 21, no. 4, pp. 343–346.
3. Oguakwa, J. U. (1984). ‘Nα-formylechitamidine, an alkaloid from Alstonia boonei’. Phytochemistry, vol. 23, no. 11, pp. 2708–2709.
4. Adotey, J.P., Adukpo G.E., Opoku Boahen, Y., Armah F.A. (2012). ‘A Review of the Ethnobotany and Pharmacological Importance of Alstonia boonei De Wild (Apocynaceae)’. ISRN Pharmacol. 2012;2012:587160. doi: 10.5402/2012/587160. Epub 2012 Jul 30. PMID: 22900200; PMCID: PMC3413980.
5. Abbiw, D. (1990). ‘Useful Plants of Ghana: West African Uses of Wild and Cultivated Plants’. Intermediate Technology Publications, Royal Botanical Garden, Kew, London.
6. Hadi, S. and Bremner, J. B., (2001). ‘Initial studies on alkaloids from Lombok medicinal plants’. Molecules, vol. 6, no. 2, pp. 117– 129.
7. Fakae, B. B., Campbell, A. M., Barrett J. et al. (2000). ‘Inhibition of glutathione S-transferases (GSTs) from parasitic nematodes by extracts from traditional Nigerian medicinal plants’. Phytotherapy Research, vol. 14, no. 8, pp. 630–634.
8. Kam, T. S. Nyeoh, K. T.. Sim, K. M and. Yoganathan, K. (1997). ‘Alkaloids from Alstonia scholaris’. Phytochemistry, 45(6):1303–1305.
9. Adote, A.J., Adukpo, G.E., Yaw, G., Armah, O.B.F. (2012). ‘A Review of the Ethnobotany and Pharmacological Importance of Alstonia boonei De Wild (Apocynaceae)’. ISRN pharmacology.. 587160. 10.5402/2012/587160.
10. Nyegue, M., Amvam-Zollo, P.H. , Etoa, F.X. , Agnaniet, H. and Menut, C. (2008). ‘Chemical and Biological Investigations of Essential Oils from Stem Barks of Enantia chlorantha Oliv. and Polyalthia suaveolens Engler. & Diels from Cameroon’. Natural Product Communications, Vol. 3 (7).
11. Gill, L.S. (1992). ‘Ethnomedical uses of plants in Nigeria’. UNIBEN Press, Benin City, 275.
12. Agbaje, E.O., Onabanjo, A.O. (1998). ‘Analgesic and antipyretic actions of Enantia chlorantha extract in some laboratory animals’. Nig J Nat Prod Med, 2: 24–25.
13. Adesokan, A.A., Akanji, M.A., and Yakubu, M.T. (2007). Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. Afr J Biotech, 6(22): 2502–2505.
14. Odugbemi, T.O., Odunayo, R.. Akinsulire, I., Aibinu, E. and Fabeku, O. (2007). ‘Medicinal Plants Useful For Malaria Therapy In Okeigbo, Ondo State, Southwest Nigeria’. Afr J Trad Cam, 4(2): 191–198.
15. Fasola, T.R., Adeyemo, F.A., Adeniji, J.A. and Okonko, I.O. (2011). ‘Antiviral potentials of Enantia chlorantha extracts on yellow fever virus’. Nat Sci, 9: 99–105.
16. Wafo, P., Nyasse, B., Fontaine, C. (1999). ‘7,8-dihydro-8-hydroxy palmitine from Enantia chlorantha’. Phytochem., 50: 279-281.
17. Ajani, E.O. and Ibrahim, L.B. (2020). ‘Toxicological evaluations of combined administration of ethanolic stem bark extract of Enantia chlorantha and lisinopril in experimental type 2 diabetes’. Clin Phytosci 6, 29.
18. Adjanohoun, J.F., Aboubakar, N., Dramane, K., Ebot, M.E., Ekpere, J.A., Enow-Orock, E.G. (1996). ‘Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanic and Floristic Studies in Cameroon’. Organization of African Unity Scientific, Technical and Research Commission. Centre Nationale de Production des Manuels Scolaires, Porto-Novo. Benin. 51: 63.
19. Obadoni, B.O., and Ochuko, P.O. (2001). ‘Phytochemical studies and comparative efficacy of the crude extract of some homeostatic plants in Edo and Delta states of Nigeria’. Global J Pure and Applied Sci, 8:203–8.
20. Siminialayi, I.M. and Agbaje, E.O. (2005). ‘Gastroprotective effects of the ethanolic extract of Enantia chlorantha in rats’. West African Journal of Pharmacology and Drug Research;20(1):35–8.
21. Madingou, N.O.K., Souza, A., Lamidi, M., Mengome, L.E., Mba, C.E.M., Bayissi, B., et al. (2012). ‘Study of medicinal plants used in the management of cardiovascular diseases at Libreville (Gabon): an ethnopharmacological approach’. Int J Pharm Sci Res, 3: 111-9.
22. Nyangono, B.C.F., Chakokam Ngangoum, R.M., Kuate, D., Ngondi, J.L., and Enyong Oben, J. (2012). ‘Effect of Guibourtia tessmannii extracts on blood lipids and oxidative stress markers in triton WR 1339 and high fat diet induced hyperlipidemic rats’. Biol Med, 4: 1-9..
23. Adjanohaon, E. J., & Ake, A. L. (1984). ‘Medecine traditionelle et phar‐macopee. Contribution aux études ethnobotaniques et floristes en République Populaire du Congo [Contribution to ethnobotanicaland floristic studies in the Popular Republic of Congo]’. Agence de Cooperation Culturelle et Technique ŽACCT.p.39. France, Paris.
24. Madingou, K. N. O., Souza, A., Lamidi, M., Mengome, L. E., Eyele, M. M. C., Bading, B. M. J., & Traore, A. S. (2012). ‘Study of medicinal plants used in the management of cardiovascular diseases at Libreville (Gabon):An ethnopharmacologicalapproach’. International Journal of Pharmaceutical Sciences and Research, 3, 111–119.
25. Defo Deeh, P.B., Watcho, P., Wankeu-Nya, M., Ngadjui, E., and Usman, Z.U. (2019). ‘The methanolic extract of Guibourtia tessmannii (caesalpiniaceae) and selenium modulate cytosolic calcium accumulation, apoptosis and oxidative stress in R2C tumour Leydig cells: Involvement of TRPV1 channels’. Andrologia., 51:e13216.
26. Fernande, N.B.C., Marthe, T., Laure, N.J., Enyong ,O.J. (2013). ‘In vitro antioxidant activity of Guibourtia tessmannii Harms, J. Leonard (Cesalpinoidae)’. J Med Plant Res, 7: 3081-8.
27. Kader, H. M., Noor, S., Radzi, M., and Wahab, N. A. A. ‘Antibacterial activities and phytochemical screening of the acetone extract from Euphorbia hirta’. International Journal of Medicinal Plant Research, vol. 2, pp. 209–214, 2013.
28. Lim, D. W., Kim, Y. T., Jang, Y. J., Kim, Y. E. and Han, D. (2013). ‘Antiobesity effect of Artemisia capillaris extracts in high-fat dietinduced obese rats’. Molecules, vol. 18, pp. 9241–9252.
29. Liu, Y., Murakami, N., Ji, H., Pedro, A. and Zhang, S. (2007). ‘Antimalarial flavonol glycosides from Euphorbia hirta’. Pharm Biol.;45:278–81.
30. Rastogi, R.P. and Mehrotra, B.N. (1998-2002) Compendium of Indian medicinal plants, Vol. 1-6. Central Drug Research Institute/National Institute of Science Communication, Lucknow/New Delhi.
31. Williamson, E.M. (2002). ‘Major Herbs of Ayurveda’. Churchill Livingstone publisher, 1st eds (October 30), ASIN: 0443072035, ISBN-10:1856174166, ISBN-13: 978-1856174169. 392 p.
32. Sood, S.K., Bhardwaj and R., Lakhanpal, T.N. (2005). ‘Ethnic Indian Plants in cure of diabetes’. Scientific Publishers, ISBN 9387741621, 9789387741621, 164 p.
33. Lanhers, M.C,. Fleurentin, J. Dorfman, P. Mortier, F. and Pelt, J.-M. (1991). ‘Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta’. Planta Medica, vol. 57, no. 3, pp. 225–231.
34. Galvez, J, Zarzuelo, A, Crespo, ME, Lorente, MD, Ocete, MA, and Jimenez, J. (1993). ‘Antidiarrheal activity of Euphorbia hirta extract and isolation of an active flavanoid constituent’. Planta Med.,59:333–36.
35. Galvez, J., Crespo, M.E., Jimenez, J., Suarez, A., and Zarzuelo, A.. ‘Antidiarrheic activity of quercitrin in mice and rats’. J Pharm Pharmacol., 1993;45:157.
36. Chopra, RN, Chopra, IC, Handa, KL, Kapur, LD. (1994). ‘Indigenous drugs of India’. Calcutta, India: Academic Publishers.
37. Kumar, S., Malhotra, R., and Kumar, D. (2010). ‘Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities’. Pharmacogn Rev. Jan-Jun; 4(7): 58–61.
38. New Delhi: Council of Industrial and Scientific Research (2005). The Wealth of India (Raw material) Vol. 3.
39. Anitha, P. Geegi, P.G. Yogeswari, J. Anthoni S.A. (2014). ‘In Vitro Anticancer Activity of Ethanolic Extract of Euphorbia hirta (L.)’. Science, Technology and Arts Research Journal1. Vol.3 No. 1 DOI:10.4314/star.v3i1.1.
40. Shih, M. F. and Cherng J.Y. (2012). ‘Potential applications of Euphorbia hirta in pharmacology’. in Drug Discovery Research in Pharmacognosy, Chapter 8, pp 165-180. Edited by Omboon Vallisuta and Suleiman M. Olimat, Published by InTech.
41. Johnson, P. B., Abdurahman, E. M., Tiam, E. A., Abdu-Aguye, I. and Hussaini, I. M. (1999). ‘Euphorbia hirta leaf extracts increase urine output and electrolytes in rats’. Journal of Ethnopharmacology,vol. 65, no. 1, pp. 63–69.
42. Gyuris, A., Szlávik, A., Minárovits, J., Vasas, A., Molnár, J. and Hohmann, J. (2009) ‘Antiviral Activities of Extracts of Euphorbia hirta L. against HIV-1, HIV-2 and SIVmac251’. In vivo 23: 429-432.
43. Perera, S. D., Jayawardena, U. A., & Jayasinghe, C. D. (2018). ‘Potential Use of Euphorbia hirta for Dengue: A Systematic Review of Scientific Evidence’. Journal of tropical medicine, 2048530.
44. Tona, L., Kambu, K., Ngimbi, N., Mesia, K., Penge, O., Lusakibanza, M., et al. (2000). ‘Antiamoebic and spasmolytic activities of extracts from some Antidiarrhoeal traditional preparations used in Kinshasa and Congo’. Phytomedicine.;7:31–8.
45. Gnecco, S., Perez, C., Bittner, M., Silva, Y.M. (1996). ‘Distribution pattern of n-alkanes in Chilean species from the Euphorbiaceae family’. Bol Soc Chil Quim., 41:229–33.
46. Kirtikar, K.R. and Basu, B.D. (2001). ‘Indian Medicinal Plants’. 2nd Edition, Oriental Enterprises, Uttaranchal, Volume 8, 2604.
47. Barua, C.C., Sen, S., Das, A.S., Talukdar, A., Jyoti Hazarika, N., Barua, A., and Barua, I. (2014). ‘A comparative study of the in vitro antioxidant property of different extracts of Acorus calamus Linn’. J. Nat. Prod. Plant Resour., 4:8–18.
48. Nićiforović, N., Mihailović, V., Mašković, P., Solujić, S., Stojković, A., and Muratspahić, D.P. (2010). ‘Antioxidant activity of selected plant species; potential new sources of natural antioxidants’. Food Chem. Toxicol., 48:3125–3130.
49. Duthie, G.G., Duthie, S.J., Kyle, and J.A.M. (2000). ‘Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants’. Nutr. Res. Rev., 13:79. doi: 10.1079/095442200108729016.
50. Li, A. N., Li, S., Zhang, Y. J., Xu, X.R., Chen, Y.M., and Li, H.B. (2014). ‘Resources and biological activities of natural polyphenols’. Nutrients, 6:6020–6047. doi: 10.3390/nu6126020.
51. Balmus, I., Ciobica, A., Trifan, A., and Stanciu, C. (2016). ‘The implications of oxidative stress and antioxidant therapies in Inflammatory Bowel Disease: Clinical aspects and animal models’. Saudi J. Gastroenterol., 22:3–17. doi: 10.4103/1319-3767.173753.
52. Miliauskas, G., Venskutonis, P.R., van Beek, T.A. (2004). ‘Screening of radical scavenging activity of some medicinal and aromatic plant extracts’. Food Chem., 85:231–237. doi: 10.1016/j.foodchem.2003.05.007.
53. Gouthamchandra, K., Mahmood, R., and Manjunatha, H. (2010). ‘Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L.’ Environ. Toxicol. Pharmacol., 30:11–18. doi: 10.1016/j.etap.2010.03.005.
54. World Health Organization (1998). ‘Quality control methods for medicinal plant materials’. Available at https://apps.who.int/iris/handle/10665/41986: 115 p. ISBN 9241545100.
55. United States Pharmacopeia (USP) and the National Formulary (NF) 35. Available at https://www.uspnf.com/official-text/proposal-statuscommentary/usp-40-nf-35. (Accessed 10 June 2020).
56. FAO, WHO Codex Alimentarius. ‘International Food standards’. (Last modified 2021). Available at https://www.fao.org/fao-who-codexalimentarius/codex-texts/maximum-residue-limits/en/. (Accessed 10 November 2021).
57. Khalid, S., Shahzad, A., Basharat, N., Abubakar, M., Anwar, P. (2018). ‘Phytochemical Screening and Analysis of Selected Medicinal Plants in Gujrat’. J Phytochemistry Biochem 2: 108.
58. Singleton, V.L. and Rossi, J.A. (1965). ‘Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagent’. Am. J. Enol. Vitic., 16, 144–158.
59. Benzie I.F.F., Strain J.J. (1996). ‘The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay’. Anal. Biochem., 239:70–76. doi: 10.1006/abio.1996.0292.
60. Prieto, P., Pineda, M., and Aguilar, M. (1999). ‘Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E’. Anal Biochem., May 1;269(2):337-41. doi: 10.1006/abio.1999.4019. PMID: 10222007.
61. Beyene, A.M., Du, X., E. Schrunk, D. et al. (2019). ‘High-performance liquid chromatography and Enzyme-Linked Immunosorbent Assay techniques for detection and quantification of aflatoxin B1 in feed samples: a comparative study’. BMC Res Notes 12, 492.
62. Ozcan, T., Akpinar-Bayizit, A., Yilmaz-Ersan, L. and Delikanli, B.. (2014). ‘Phenolics in Human Health’. International Journal of Chemical Engineering and Applications, Vol. 5, No. 5, October
63. González-Gallego, J., García-Mediavilla, M.V., Sánchez-Campos, S., Tuñón, M.J. (2010). ‘Fruit polyphenols, immunity and inflammation’. Br J Nutr, 104(Suppl 3):S15–27.
64. Somerville, V. S., Braakhuis, A. J., & Hopkins, W. G. (2016). ‘Effect of Flavonoids on Upper Respiratory Tract Infections and Immune Function: A Systematic Review and Meta-Analysis’. Advances in nutrition (Bethesda, Md.), 7(3), 488–497.
65. Kaul, T.N., Middleton, E., and Ogra, P.L.. (1985). ‘Antiviral effect of flavonoids on human viruses’. J Med Virol;15:71–9.
66. Béládi, I., Pusztai, R., Mucsi, I., Bakay, M., and Gábor, M. (1977). ‘Activity of some flavonoids against viruses’. Ann N Y Acad Sci, 284:358–64.
67. Hämäläinen, M., Nieminen ,R., Vuorela, P., Heinonen, M., and Moilanen, E. (2007). ‘Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages’. Mediators Inflamm., 2007:45673.
68. Peluso, I., Miglio, C., Morabito, G., Ioannone, F., and Serafini, M. (2015). ‘Flavonoids and immune function in human: a systematic review’. Crit Rev Food Sci Nutr, 55:383–95.
69. Cox-Georgian, D., Ramadoss, N., Dona, C., & Basu, C. (2019). ‘Therapeutic and Medicinal Uses of Terpenes’. Medicinal Plants: From Farm to Pharmacy, 333–359.
70. Brook, K., Bennett, J., Desai, S.P. (2017). ‘The Chemical History of Morphine: An 8000-year Journey, from Resin to de-novo Synthesis’. J. Anesth. Hist., 3:50–55. doi: 10.1016/j.janh.2017.02.001.
71. Cushnie, T.P.T., Cushnie, B., Lamb, A.J. (2014). ‘Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities’. Int. J. Antimicrob. Agents, 44:377–386. doi: 10.1016/j.ijantimicag.2014.06.001.
72. Khan, H., Mubarak, M.S., and Amin, S. (2017). Antifungal Potential of Alkaloids As An Emerging Therapeutic Target. Curr. Drug Targets, 18 doi: 10.2174/1389450117666160719095517.
73. Thompson, P.L. and Nidorf, S.M. (2018). ‘Colchicine: An Affordable Anti-Inflammatory Agent for Atherosclerosis’. Curr. Opin. Lipidol.;29:467–473.
74. Manayi, A., Nabavi, S.M., Setzer, W.N., Jafari, S. (2019’). Piperine as a Potential Anti-cancer Agent: A Review on Preclinical Studies’. Curr. Med. Chem., 25:4918–4928. 75.
75 Xu, W., Zhang, M., Liu, H., Wei, K., He, M., Li, X., Hu, D., Yang, S., and Zheng, Y (2019). ‘Antiviral activity of aconite alkaloids from Aconitum carmichaelii Debx’. Nat. Prod. Res., 33:1486–1490.
76. Hung, T.C., Jassey, A., Liu, C.-H., Lin, C.-J., Lin, C.-C., Wong,S.H., Wang J.Y., Yen M.-H., and Lin L.-T. (2019). ‘Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein’. Phytomedicine, 53:62–69. doi: 10.1016/j.phymed.2018.09.025.
77. Luganini, A., Mercorelli, B., Messa, L., Palù, G., Gribaudo, G., Loregian, A. (2019). ‘The isoquinoline alkaloid berberine inhibits human cytomegalovirus replication by interfering with the viral Immediate Early-2 (IE2) protein transactivating activity’. Antivir. Res., 164:52–60. doi: 10.1016/j.antiviral.2019.02.006.
78. Varghese, F.S., Thaa, B., Amrun, S.N., Simarmata, D., Rausalu, K., Nyman, T.A., Merits, A., McInerney, G.M., Ng, L.F.P., and Ahola T. (2016). ‘The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling’. J. Virol., 90:9743–9757. doi: 10.1128/JVI.01382-16.
79. Diosa-Toro, M., Troost, B., van de Pol, D., Heberle, A.M., Urcuqui-Inchima, S., Thedieck, K., and Smit, J.M. (2019). ‘Tomatidine, a novel antiviral compound towards dengue virus’. Antivir. Res., 161:90–99. doi: 10.1016/j.antiviral.2018.11.011.
80. McMahon, J.B., Currens, M.J., Gulakowski, R.J., Buckheit, R.W., Lackman-Smith, C., Hallock ,Y.F., and Boyd, M.R. (1995). ‘Michellamine B, a novel plant alkaloid, inhibits human immunodeficiency virus-induced cell killing by at least two distinct mechanisms’. Antimicrob. Agents Chemother., 39:484–488. doi: 10.1128/AAC.39.2.484.
81. Dai, J.P., Wang, Q.W., Su, Y., Gu, L.-M., Deng, H.-X., Chen, X.-X., Li, W.-Z., and Li, K.-S. (2018). ‘Oxymatrine Inhibits Influenza A Virus Replication and Inflammation via TLR4, p38 MAPK and NF-κB Pathways’. Int. J. Mol. Sci., 19:965. doi: 10.3390/ijms19040965.
82. Fielding, B. C., da Silva Maia Bezerra Filho, C., Ismail, N., & Sousa, D. P. (2020). ‘Alkaloids: Therapeutic Potential against Human Coronaviruses’. Molecules (Basel, Switzerland), 25(23), 5496.
83. Li, S., Chen, C., Zhang, H., Guo, H., Wang, H., Wang, L., Zhang, X., Hua, S., Yu, J., and Xiao, P. (2005). ‘Identification of natural compounds with antiviral activities against SARS-associated coronavirus’. Antivir. Res., 67:18–23. doi: 10.1016/j.antiviral.2005.02.007.
84. Kim, D., Min, J., Jang, M., Lee, J., Shin, Y., Park, C., Song, J., Kim, H., Kim, S., Jin, Y.-H., et al. (2019). ‘Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells’. Biomolecules.;9:696. doi: 10.3390/biom9110696.
85. Chung, K.T., Wong, T.Y., Wei, C.I., Huang, Y.W., and Lin, Y. (1998). ‘Tannins and human health: a review’. Crit Rev Food Sci Nutr. Aug;38(6):421-64. doi: 10.1080/10408699891274273. PMID: 9759559
86. Venugopala, K. N., Rashmi, V., and Odhav, B. (2013). ‘Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity’. BioMed Research International, Article ID 963248, 14 pages.
87. Kumar, N., & Goel, N. (2019). ‘Phenolic acids: Natural versatile molecules with promising therapeutic applications’. Biotechnology reports (Amsterdam, Netherlands), 24, e00370.
88. Okwu, D.E. and Okwu, M.E. (2004). ‘Chemical composition of Spondias mombin Linn. Plant parts. J. Sustain. Agric Environ., 6 (2): 140-147.
89. Nigam M. (2021). ‘Phytomedicine: Scope and current highlights. Preparation of Phytopharmaceuticals for the Management of Disorders’. Academic Press, Chapter 3. Pages 39-54, ISBN 9780128202845.
90. Akinmoladun, A.C., Ibukun, E.O., Afor, E., Akinrinlola, B.L., Onibon, T.R, Akinboboye, A.O. et al. (2007). ‘Chemical constituents and antioxidant activity of Alstonia boonei’. Afr. J.Biotechnol., 6(10): 1197-1201.
91. Liu, R.H. (2004). ‘Potential synergy of phytochemicals in cancer prevention: mechanism of action’. J. Nutr., 134: 3479S-3485S.
AUTHOR INFORMATION
1Chemistry Advanced Research Centre, Sheda Science and Technology Complex Abuja, Nigeria.
2Institute of Medical Research and Studies of Medicinal Plants, IMPM, Yaoundé, Cameroon
3 Department of Public Health, King David University of Medical Sciences, Faculty of Allied Health Sciences, Uburu. Ebonyi State, Nigeria
4Department of Biochemistry, Faculty of Medicine and Biological Sciences, University of Yaoundé I, Cameroon
5University Institute Joseph Ndi-Samba, Yaoundé, Cameroon