Acute toxicity assessment of the STD Tea herbal remedy containg E. Chlorantha, P. nitida, E. hirta, C. papaya, M. Africana and traditionally used to treat typhoid, amoebiasis, food poisoning and urogenital infections
Cite: Pieme, C.A., Nitcheu-Sudeu, I.L., Ngouyem-Dehayem, P.1, Edoun, F.E.L., Nwaze, E.O. and Marlyse M. Peyou-Ndi. 'Acute toxicity assessment of the STD Tea herbal remedy containg E. Chlorantha, P. nitida, E. hirta, C. papaya, M. Africana and traditionally used to treat typhoid, amoebiasis, food poisoning and urogenital infections'. The Int. J. Holist. Sci. Innov. Dev., 11(3), pp. 115–131.
Acute toxicity assessment of the STD Tea herbal remedy containg E. Chlorantha, P. nitida, E. hirta, C. papaya, M. Africana and traditionally used to treat typhoid, amoebiasis, food poisoning and urogenital infections
Constant A. Pieme1, Irina L. Nitcheu-Sudeu1, Patricia Ngouyem-Dehayem1, Ferdinand E.L. Edoun2, Eric O. Nwaze3 and Marlyse M. Peyou-Ndi1,4
Corresponding author: Irina Nitcheu-Sudeu, irinasudeu@yahoo.com - Accepted: October 2022 - Published: November 2022ABSTRACT
The majority of the African population uses traditional medicine for primary care. The rise of resistance to antimicrobials calls for a permanent design of additional drugs. STD tea is an indigenous medicine containing E. Chlorantha, P. nitida, E. hirta, C. papaya, and M. Africana. The product is used traditionally to address typhoid, diarrhea, food poisoning, amoebiasis, urogenital and respiratory infections. Even though the toxicity of its ingredients had been studied, there was no published indication on its potential inherent toxicity. To uncover the safety of this product, its acute toxicity was examined using male and female albino rats; 15 female and 15 male rats divided in 6 groups were treated with STD tea orally at 2000 and 5000 mg /kg of body weight for two weeks, doses that are 40 to 100 times the recommended human doses, respectively. Rats treated with distilled water were used as controls. Mortality, clinical signs, body weight changes, food and water consumption, urinalysis, hematological and biochemical parameters, organ weights and histological markers were monitored during the study period. We found no abnormality in clinical signs, body weight, and histology results for any of the animals in the acute study following oral administration of STD tea up to 2000 mg/kg of body weight (bw) and no death were recorded up to 5000 mg/kg bw. The lethal dose with a 50% mortality rate (LD50) was estimated over 5000 mg/kg bw. These studies indicate that the ethnomedical use of STD tea is safe when used at prescribed doses.
Key words: STD Tea, typhoid, bacterial infections, food poisoning, amoeba, urogenital infections
I. INTRODUCTION
Hundreds of thousands of lives are being lost every year because of bacterial and parasitic infections that can no longer be treated with existing drugs. Discovering new antibiotics and anti-parasitic drugs including plant based drugs, able to kill drug-resistant bacteria, is essential for successful comprehensive modern medicine.
Herbal medicine has always represented an important component of primary health care. It is estimated that approximately 80% of the world’s population uses herbal medicinal products for their therapeutic virtues [1-3]. The industry of plant based drugs is believed to be experiencing a high growth rate. In the context of a high demand from consumers, there is an increased pressure to assess the efficacy of plant products. Besides, some plants may have a potentially inherent toxicity while some may be contaminated by pesticides, heavy metals or microorganisms. There is therefore a growing need to ensure the safety of phytomedicines. To address this aspect, a toxicological assessment is required to eliminate potential safety concerns.
Several medicinal plant products have been used as category I improved traditional medicines for their antibacterial an antiparasitic activities, including STD Tea and its main ingredients, Carica papaya [4], Enantia chlorantha [5], E. hirta, M. Africana. The safety of the latter and several other plants have been scrutinized. It was reported that the aqueous extract of C. papaya leaves given to pregnant rats showed some defects in fetus [6]. Acute toxicity studies which involved a single dose administration up to 2 g/kg of body weight (bw) of C. papaya leaf juice to rats, followed by a fourteen-day observation, showed dehydration demonstrated by an increase in red cell mass [7]. A repeated dose 28-day oral toxicity study of the leaf extract in rats indicated that the plant extract did not cause mortality, there were no treatment-related changes, and all organs revealed no morphological alterations [8]. During some other studies, the administration of rats with low, medium, and high doses (0.01, 0.14, and 2 g/kg bw, respectively) of fresh juice of C. papaya leaf extract for 13 weeks did not cause any changes in body weight, food intake, and water level. There were also no significant differences observed in hematology parameters between treatment and control groups. However, there were significant differences in biochemistry values, such as the LDH, creatinine, total protein, and albumin. Nevertheless, these changes were not associated with histopathological changes and were not dose dependent. Such finding on possible liver dysfunction needed to be confirmed with appropriate hepatotoxicity study protocol to elucidate extension of the toxic effect if any [9].
The therapeutic application of the extract of E. chlorantha had been shown to be quite safe at doses below 1000 mg/kg bw, suggesting that oral application of E. chlorantha may not produce toxic effects at doses lower than 500 mg extract/kg bw [10]. Other studies consider extracts of E. chlorantha, to be nontoxic in acute intake up to 5000 mg/kg bw, meaning a LD50 >5000 mg/kg bw [11], since the extract at a single dose of 5,000 mg/kg bw did not produce treatment related signs of toxicity in rats or mortality during a 14-day observation period.
In acute and sub-chronic studies, the LD50 of E. hirta was estimated to be over 5,000 mg/kg bw [12]. In the repeated dose 90-day oral toxicity study, the administration of 50, 250, and 1000 mg/kg bw/day of E. hirta extract revealed no significant difference in food and water consumptions, body weight change, hematological and biochemical parameters, relative organ weights, and gross findings compared to the control group. Macropathology and histopathology examinations of all organs including the liver did not reveal morphological alterations. Analyses of these results with the information of signs, behaviour, and health monitoring indicated that the long-term oral administration of E. hirta extract for 90 days does not cause sub-chronic toxicity [12].
In previous studies [13], mice were treated intraperitoneally with M. Africana doses ranging from 50 to 1000mg/kg of the crude extract. The intraperitoneal LD50 was calculated to be 387.3 mg/kg bw. Oral administration of ethanolic stem bark extract of M. africana (30-90 mg/kg) for 21 days did not produce deleterious alteration in the levels of Alkaline phosphatase (ALP), Alanine transaminase (ALT) and Aspartate transaminase (AST) of the extract treated rats compared to control, even though a significant decrease in ALP and AST levels was observed. There was a dose dependent significant decrease in the levels of total protein and albumin. The administration of the extract did not produce considerable changes in the hematological parameters of extract treated rats, except a significant increase in withe blood cell counts which was dose dependent [13].
Although reports of scientific studies on STD Tea plant ingredients have been published, there is no published information on its inherent safety. Besides, there is no record in the literature involving herbal remedies containing a mixture of the above mentioned plants. We set out to identify possible adverse effects from this remedy consumption on hematological, histological and biochemical parameters using rats. The purpose being to determine whether STD Tea has the potential to be toxic to biological organisms and, if so, to what extent.
II. MATERIALS AND METHODS
2.1. Experimental Animals
White alibino rats with a weight varying between 100 and 250 g were obtained from the local animal house of the Institute of Medical Research and Medicinal Plants Studies in Cameroon. Mice were acclimatized to standard laboratory conditions for 7 days. Institutional animal ethical guidelines were strictly observed.
2.2. Plant material, equipment and reagents
STD tea is a pure natural plant product manufactured by Reece International Research Consortium, with plants grown in Cameroon including Carica papaya, Enantia chlorantha , E. hirta and M. Africana, with equipment mainly manufactured in the USA, most donated by Oregon Health Sciences University and the National Insurance Funds of Cameroon. Chemicals used for the studies were of analytical grade and sourced from the USA. The remedy is classified as a category I improved traditional medicine, mainly used in traditional or complementary medicine to fight bacterial and parasitic infections.
2.3. Acute toxicity
Acute toxicity testing was carried out at the University of Yaounde I, Faculty of Medicine and Biomedical sciences, Department of Biochemistry and Physiology, and at the University Institute Ndi-Samba. Acute toxicity studies where performed according to the OECD Guideline 420 basics: Acute Oral Toxicity - Fixed Dose Procedure was used. In this procedure, the initial dose level was selected on the basis of a sighting study as the dose expected to produce some signs of toxicity without causing severe toxic effects or mortality. Further groups of animals were dosed at higher or lower fixed doses, depending on the presence or absence of signs of toxicity or mortality. This procedure continued until the dose causing evident toxicity or death was identified, or when no effects were seen at the highest dose or when deaths eventually occured at the lowest dose.
Thirty albino rats (15 males and 15 females) with a weight varying between 150 and 200 g were divided into 6 groups of five animals each: Healthy female control (5 mice which received distilled water), Female test (5 mice who received the improved traditional medicine STD Tea at the dose of 2000 mg and 5000 mg per Kg of body weight respectively), Healthy male control (5 mice who received distilled water), Male test (5 mice which received STD Tea at the dose of 2000 mg and 5000 mg per kg of body weight, respectively). The test remedy was administered in a single dose by gavage using a stomach tube. Animals were fasted prior to dosing. The following parameters over a period of 14 days were evaluated: LD50, general behavior of animals (aggressiveness, excessive cleaning, drowsiness, immobility, etc.); zootechnical criteria (food intake, water intake, weight change and weight gain); Macroscopic and microscopic observation of internal organs of animals after 14 days, in particular the heart, liver, lungs, kidneys, brain, spleen, testis or ovary: Histological analysis of target organs (liver, kidneys, lungs and heart); and Biochemical analysis of toxicity markers (AST, ALT, Triglycerides, Total proteins and Creatinine). Information was collected on the hazardous properties that would allow STD tea to be classified for acute toxicity according to the Globally Harmonized System of classification and labelling of chemicals.
From the result of an acute toxicity test, a conclusion can be made on the toxicity status of the test substance. Substances with LD50 below 5 mg/ kg are classified to be extremely toxic, 5–50 mg/kg - highly toxic, 50–500 mg/kg - moderately toxic, 500–5,000 mg/kg - slightly toxic, 5000-15,000 mg/kg - Practically non-toxic, and > 15,000 mg/kg bw - relatively harmless [14]. The tests were done using the 190-1100 Single beam UV/VIS spectrophotometer.
2.4. Statistical analysis
Data were expressed as mean ± standard deviation. Statistical analysis was performed using GraphPad Prism version 5.00 for windows in which one-way ANOVA was used followed by Waller Duncan multiple comparison tests at p < 0:05 and/or the Turkey-Kramer post hoc multiple comparison test.
III. RESULTS
3.1. Total weight gain of animals subjected to the administration of the STD Tea product
The kinetics of the weight gain were monitored and total weight gain of the control groups was compared to that of the STD Tea treated group following a 14-day observation period. The remedy caused a non-significant increase, with a p-value > 0.05, in the weight gain of animals in the test groups compared to those of the control groups (Figures 1 and 2). No deaths were recorded in test animals at all doses, from 2000mg/kg to 5000 mg/kg bw. The LD50 was evaluated as being over 5000 mg/Kg bw.
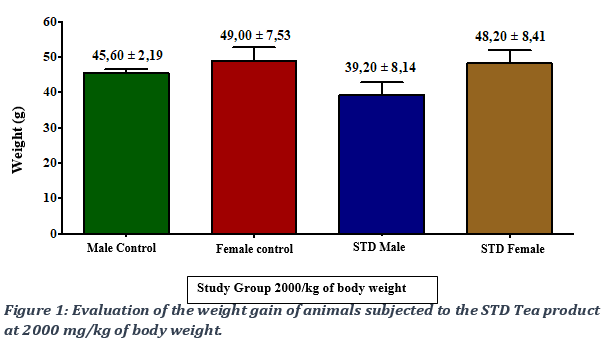
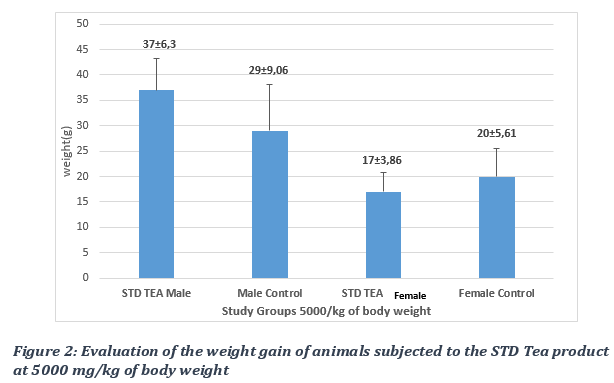
3.2. Evaluation of food intake following administration of the STD Tea product.
When comparing the food consumption of animals subjected to a single-dose treatment of the STD TEA product at 2000 mg/kg of body weight with that of the control group, we observed a non-significant difference in food intake between the two groups with a p- value > 0.05 (Figure 3).
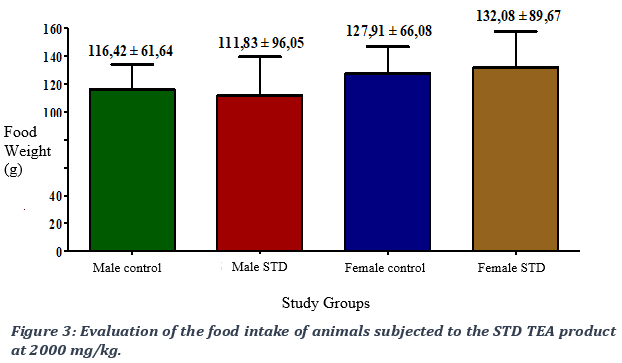
3.3. Evaluation of water intake following administration of the STD TEA product.
In terms of water intake, a non-significant decrease in water intake was observed with a p-value > 0.05 in test animals administered the STD TEA product in both male and female compared to control groups (Figure 4).
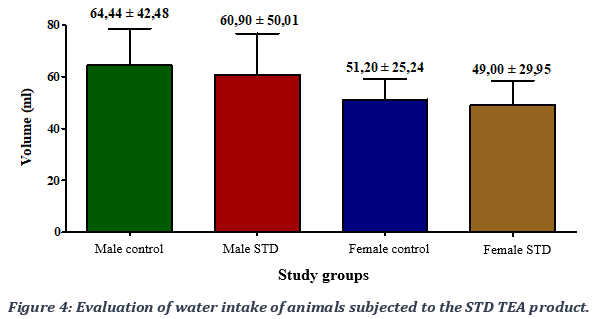
3.4. Evaluation of the effect of the STD TEA product on the relative weight of animals’ internal organs
The effect of the STD tea at 2000mg/kg bw (Table 1) and 5000mg/kg bw (Table 2) on the relative weight of internal organs of rats was investigated. No significant differences were observed, with a p-value > 0.05, between untreated and treated animals. The product therefore does not affect the internal macroscopy of the animals.
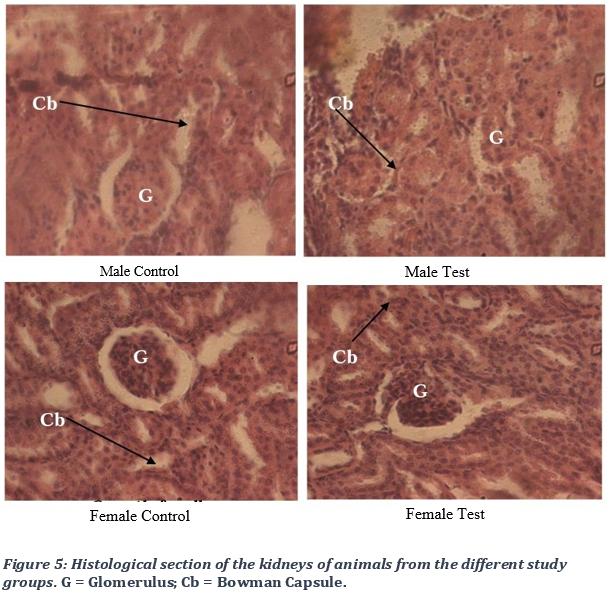
3.5. Evaluation of the effect of STD Tea on rat liver and kidney histology
Kidney structure analysis
The histological analysis of the rat kidneys in different study groups, rats treated with the remedy (test group) and untreated rats(control), did not show any morphological alteration of the internal structure of the kidney, whether at the level of the glomerulus, the Bowmann capsule, the urinary space and the podocytes are well diversified (Figure 5).
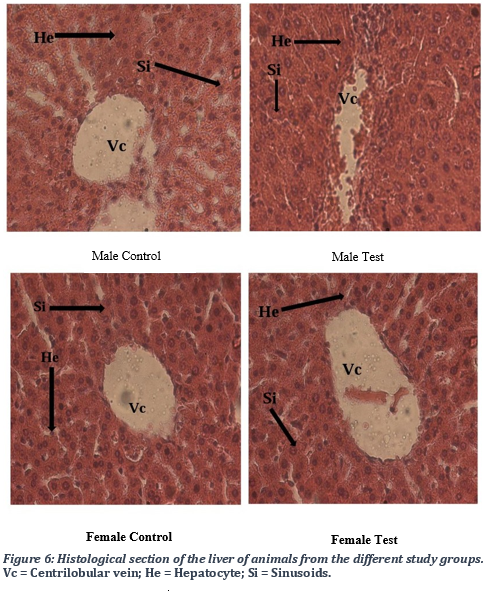
Analysis of the liver structure
Histological analysis of the liver of different groups of animals, those treated with the remedy (test group) or untreated (control), did not show any major changes in the internal structure of the liver. The analysis of micrographs revealed a normal architecture of the centrilobular vein, and in hepatocytes in both control and the test groups. Overall, there was an absence of major pathological alterations.
3.6. Evaluation of the effect of STD Tea on hepatic and renal biochemical parameters.
Effects of the remedy on hepatic cytolysis
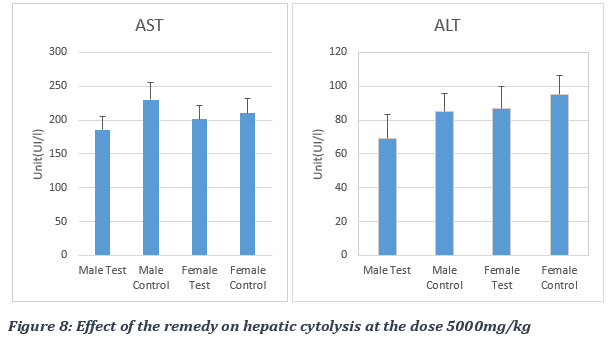
Increased serum level of ALT and AST is commonly used as a biomarker of hepatocellular injury, though its elevation can also be typical of muscle and cardiac damage, respectively. The administration of the remedy did not produce significant different levels of ALT, which is specific for liver and kidney cells (Figure 7). The results obtained at the dose of 2000 mg/kg bw were: 98.30 ± 22.37 IU/l for the male control group, 96.98 ± 09.04 IU/L for the male test group, 84.90 ± 26.32 IU/L for the female control group and 83.14 ± 10.33 IU/L for the female test group (Figure 7). At dose of 5000 mg/kg bw, results of ALT level were 85.30 ± 11.07 IU/l for the male control group, 68.87 ± 14.7 IU/L for the male test group, 87.90 ± 13 IU/L for the female test group and 95.09 ± 11 IU/L for the female control group (Figure 8). We therefore observed a non-significant decrease of the enzymatic level following the administration of the plant preparation with a p-value > 0.05.
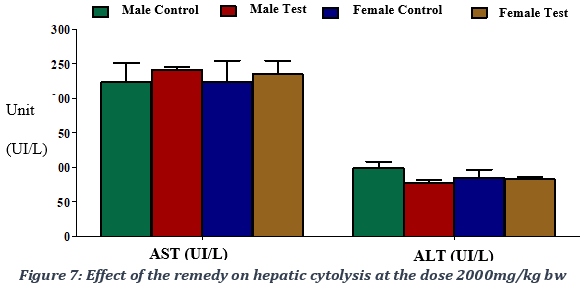
For rats treated with 2000 mg of the remedy /kg bw, AST values were 246.80 ± 27.58 IU/L for the male test group versus 223.60 ± 62.65 IU/L for the male control group, 239.80 ± 27.45 IU/L for the female test group versus 224.00 ± 68.84 IU/L for the female control group. As for the 5000 mg remedy /kg bw experimental group, AST values were 185,87± 19 IU/L for the male test group versus 230± 125 IU/L for the male control group, 200.98 ± 20 IU/L for the female test group versus 210.00 ± 22 IU/L for the female control group. The administration of the remedy produced a non-significant elevation, with a p-value > 0.05, of the AST enzyme marker activity, which is an enzyme specific to skeletal and cardiac muscles. The AST/ALT ratio of approximately 1 indicated an absence of liver damage following the administration of the STD tea product (Figure 7).
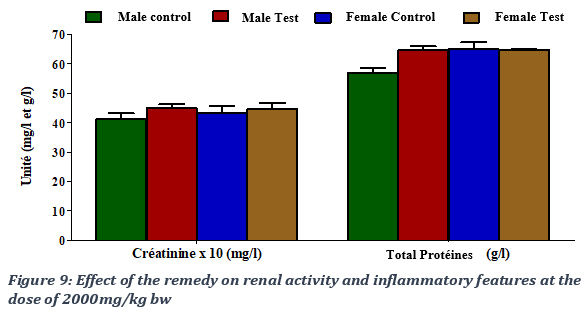
Effects of the remedy on renal and inflammatory parameters
Creatinine is the most commonly used indicator of renal function. At 2000 mg of remedy administered /kg of rat body weight, obtained creatinine values were 4.11 ± 0.45 mg/l for the male control group, 4.67 ± 0.59 mg/l for the male test group, 4.34 ± 0.51 mg/l for the female control group and 4.45 ± 0.29) mg/l for the female test group. The remedy produced a non-significant increase creatinine, with a p-value > 0.05. Low protein level may indicate a liver or kidney problem, or it may be that protein isn't being digested or absorbed properly. A high total protein level could indicate dehydration, certain chronic inflammation or inflammatory disorders or some types of cancer. The administration of the remedy resulted in a non-significant increase in the level of total proteins in the male group at 2000 mg/kg bw with a p-value > 0.05; total proteins’ values were estimated at 56.94 ± 3.3) g/l for the male control group, 64.72 ± 2.77) g/l for the male test group, 65.02 ± 3.88 g/l for the female control group and 64.54 ± 1.35 g/l for the female test group (Figure 9).
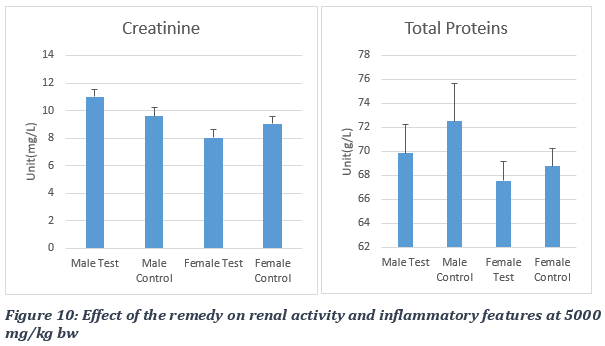
During the experiment using the remedy dose of 5000 mg/kg of rat body weight, the administration of the STD product produced a non-significant increase in creatinine levels in the male test group, with a p-value > 0.05. The creatinine marker values obtained were 9.6 ± 0.53 mg/l for the male control group, 11 ± 0.53 mg/l for the male test group, 9.05± 0.55 mg/l for the female control group and 8.02 ± 0.59 mg/l for the female test group (Figure 10). The administration of the plant product at 5000 mg/kg bw produced a non-significant decrease in the level of total proteins in treated (test) groups with a p-value > 0.05. The values obtained were 72.50 ± 3.14 g/l for the male control group, 69.85 ± 2.42 g/l for the male test group, 68.78 ± 1.44 g/l for the female control group and 67.54 ± 1.60) g/l for the female test group (Figure 10).
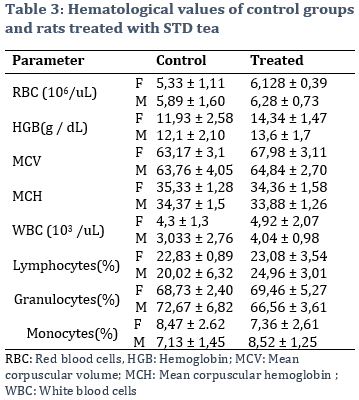
3.7. Evaluation of the effect of the STD Tea on hematological parameters.
Hematological parameters were compared between control groups and rats administered 5000 mg of the remedy per kg of body weight. Results showed an increase of white and red blood cells, and of hemoglobin values for both female and male tests groups, respectively. However, these changes were not evaluated as being significant (Table 3).
VI. DISCUSSION
Toxicity assessment through biochemical tests and histological analysis is necessary to establish how safe a medication is for human consumption. It indicates the potential of drugs under specific doses to cause serious toxic effect or not. The harmonized toxicity evaluation uses LD50 as a quick valuable tool. Data in the literature show that LD50 varies depending on the animal and the route of administration into the body of laboratory animals. The oral LD50 of STD tea in rats was estimated to be over 5000 mg/kg of body weight, allowing the product to be initially classified as practically non-toxic. Daily administration of STD tea high doses to rats for 2 weeks did not cause any significant change in body weight, food intake, and water level. There were also no significant differences observed on hematology parameters between treated and control groups. There was absence of pathological signs at high doses for no evidence of renal or hepatic toxicity was found upon histological analysis of the liver and kidneys in different groups under study, as shown in previous single ingredient studies [8,12]. No major changes in the internal structure of these major organs was observed. In general, in acute parenchyma liver damage, alanine aminotransferase (ALT) is elevated. The determination of ALT activity is an indicator of liver damage. Aspartate aminotransferase (AST) has a role similar to ALT, but is found in various tissues and organs such as liver, heart, brain, lungs, pancreas and muscles. During the study, administration of the remedy did not lead to an elevation of ALT at doses 40 times higher than the therapeutic dose. When administered doses 100 times the recommended dose, there was a non-significant decrease of the enzyme level following the administration of the remedy, thus confirming the absence of hepatotoxicity even at high doses used. The remedy led to a non-significant elevation of AST at doses 40 times the therapeutic dose and a non-significant decrease of the same marker at doses 100 times higher than the therapeutic dose. The AST/ALT ratio indicated an absence of liver damage following the administration of the traditional medicine.
Besides, measuring serum creatinine is the most commonly used indicator of renal function. A rise in blood creatinine concentration is a late marker observed with marked damage to functioning nephrons. The administration of the remedy produced a non-significant increase in renal activity at high doses, and did not cause an inflammatory reaction, translated by a non-significant increase in the level of total proteins. While some studies reported significant non-dose-dependent decrease in creatinine and increase in total protein levels when C. papaya, one of the STD Tea ingredients, was administered [7-9], we failed to observe the same results with the remedy feeding, even at very high doses. However, that difference could eventually be attributed to the dilution effect provided by the presence of other ingredients. Indeed, other studies demonstrated that oral administration of M. Africana produce a dose dependent significant decrease in the levels of total protein and a significant decrease in AST levels [13]. As previously stated, we instead observed a non-significant elevation of AST in rats treated with high doses of STD Tea. Moreover, it was reported that administration of M. Africana extract produced a significant decrease in withe blood cell count which was dose dependent [13]. We did observe an increase in both white and red blood cells instead, but the changes were estimated not significant. More studies are needed to ascertain these findings, for literature data indicate that while some ingredient may not be toxic in acute intake, high doses can cause lungs, hepatic and kidney disorders following medium to long-term use [15].
To conclude, there was absence of death following the administration of the product at 2000 and 5000 mg per kg of body weight. There were no pathological signs following histology and markers’ analysis. The product is classified practically non-toxic. However, results could be completed with additional toxicity experiments, general toxicity studies normally including acute toxicity, subacute toxicity, and chronic or subchronic toxicity studies.
ACKNOWLEDGEMENTS
Financial support by the National Social Insurance Fund (CNPS) and its Director Mr Mekulu Mvondo Alain Olivier is highly appreciated. We thank Oregon Health Sciences University for equipment donation by the Former President Mr Jim Walker. The participation of Reece International Research Consortium (RIRCO) researchers in the development of endogenous solutions and subsequent research is highly appreciated.
CONFLICT OF INTEREST
RIRCO is a not for profit community based organization promoting research and the development of improved science based natural indigenous medicines to help fight epidemics and global public health threats. RIRCO researchers provide assistance in both the development of improved traditional medicines and related research in resource limited settings.
REFERENCES
1. Woo, C.S.J., Lau, J.S.H., and El-Nezami, H. (2012). Herbal medicine: Toxicity and recent trends in assessing their potential toxic effects. In Advances in Botanical Research; Shyur, L.-F. Lau A.S.Y. Eds. Elsevier Academic Press: Cambridge, MA, USA; Volume 62, pp. 365–384.
2. Islam, S.U., Dar, T.U., Khuroo, A.A., Bhat, B.A., Mangral, Z.A., Tariq, L., Tantray, W.W., and Malik, A.H. (2021). ‘DNA barcoding aids in identification of adulterants of Trillium govanianum Wall. ex D’. Don. J. Appl. Res. Med. Aromat. Plants, 23, 100305.
3. Aware, C.B., Patil, D.N., Suryawanshi, S.S., Mali, P.R., Rane, M.R., Gurav, R.G., and Jadhav, J.P. (2022). ‘Natural bioactive products as promising therapeutics: A review of natural product-based drug development’. S. Afr. J. Bot., 151, 512–528.
4. Nkuo-Akenji T., Ndip R., McThomas A., and Fru E.C. (2001). “Anti-Salmonella activity of medicinal plants from Cameroon’. Cent Afr J Med. Jun;47(6):155-8. doi: 10.4314/cajm.v47i6.8607. PMID: 12201022.
5. Ebelle Etame R., Mouokeu R.S., Cidjeu Pouaha C.L., Voukeng Kenfack I., Tchientcheu R., Assam Assam J.P., Monthe Poundeu F.S., Tchinda Tiabou A., Etoa F.X., Kuiate J.R., and Ngono Ngane R.A. (2018). ‘Effect of Fractioning on Antibacterial Activity of Enantia chlorantha Oliver (Annonaceae) Methanol Extract and Mode of Action’. Evid Based Complement Alternat Med. Apr 29;2018:4831593. doi: 10.1155/2018/4831593. PMID: 29853954; PMCID: PMC5949159.
6. Ekong M. B., Akpan M. U., Ekanem T. B., and Akpaso M. I. (2011). ‘Morphometric malformations in fetal rats following treatment with aqueous leaf extract of Carica papaya’. Asian Journal of Medical Sciences ;2(1):18–22.
7. Halim S. Z., Abdullah N. R., Afzan A., Abdul Rashid B. A., Jantan I., and Ismail Z. (2011). ‘Study of acute toxicity of Carica papaya leaf extract in Sprague Dawley rats’. Journal of Medicinal Plants Research ;5(10):1867–1872.
8. Afzan A., Abdullah N. R., Halim S. Z., Rashid B. A., Semail R. H. R., Jantan I., Muhammad H., and Ismail Z. (2012). ‘Repeated dose 28-days oral toxicity study of Carica papaya L. leaf extract in Sprague Dawley rats’. Molecules;17(4):4326–4342. doi: 10.3390/molecules17044326.
9. Ismail Z., Halim S.Z., Abdullah N.R., Afzan A., Abdul Rashid B.A., and Jantan I. (2014). ‘Safety Evaluation of Oral Toxicity of Carica papaya Linn. Leaves: A Subchronic Toxicity Study in Sprague Dawley Rats’. Evid Based Complement Alternat Med. ;741470. doi: 10.1155/2014/741470. Epub Oct 29. PMID: 25530788; PMCID: PMC4228719.
10. Adebiyi O.E. and Abatan M.O (2013). ‘Phytochemical and acute toxicity of ethanolic extract of Enantia chlorantha (oliv) stem bark in albino rats’. Interdiscip Toxicol. Sep;6(3):145-51. doi: 10.2478/intox-2013-0023. PMID: 24678252; PMCID: PMC3967441.
11. Tan, P.V., Boda, M., Enow-Orock, G., Etoa, F., and Bitolog, P. (2007). ‘Acute and sub-acute toxicity profile of the aqueous stem bark extract of enantia chlorantha oliver (annonaceae) in laboratory animals’. Pharmacologyonline 1: 304-313.
12. Yuet Ping K., Darah I., Chen Y., Sreeramanan S., and Sasidharan S. (2013). ‘Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats’. Biomed Res Int. 2013;2013:182064. doi: 10.1155/2013/182064. Epub 2013 Dec 10. PMID: 24386634; PMCID: PMC3872372.
13. AntiaB. S., Okokon J. E., 3Nwidu L. L. and Jackson C.L. ((2006). ‘Effect of Subchronic Administration of Ethanolic Stembark Extract of Mammea Africana Sabine on Haematological and Biochemical Parameters of Rats’. African Journal of Biomedical Research, Vol. 9; 129 -132.
14. Loomis, T.A., and Hayes, A.W. (1996). Loomis’s essentials of toxicology (Fourth 4 Edition). pp. 208–245. California: Academic press.
15. Akinwale S.G., Chukwu O.E., Chioma O.P., Chukudi A.J. and Olubunmi A.G. (2022). ‘Enantia chlorantha: A review’. J Pharmacogn Phytochem;11(3):34-38. DOI: 10.22271/phyto.2022.v11.i3a.14406.
AUTHOR INFORMATION
1Department of Biochemistry, Faculty of Medicine and Biomedical Sciences, University of Yaoundé I, Cameroon
2Institute of Medical Research and Medicinal Plant Studies, Yaounde, Cameroon.
3Department of Public Health, Faculty of Allied Health Sciences, King David University of Medical Sciences, Uburu, Ebonyi State, Nigeria
4Reece International Research Consortium, University Institute Ndi-Samba, Yaounde, Cameroon